(396b) Deactivation Study of Mo-HZSM-5 Catalyst for Microwave-Assisted Methane Aromatization: Effect of Reaction Parameters
AIChE Annual Meeting
2022
2022 Annual Meeting
Fuels and Petrochemicals Division
Value-Added Chemicals from Natural Gas I
Tuesday, November 15, 2022 - 3:49pm to 4:08pm
Methane is known as the primary constituent of natural gas. Direct catalytic conversion of methane to benzene using methane aromatization (MDHA) can convert methane directly to benzene and hydrogen[2] (eqn. 1).
6CH4 = 4C6H6 + 9H2 ; (ΔG298K = +433 kJ/mol, ΔH298K = +530 kJ/mol) (1)
Very high temperatures (700ºC-1000ºC) are needed to drive the equilibrium towards the formation of benzene and hydrogen. However, challenges of rapid catalyst coking along with thermodynamic limitations represent a major challenge towards a process with high conversions and product yields[3]. Mo/HZSM-5 catalyst has been demonstrated as the most active/selective catalyst for MDHA due the proximity of molybdenum to internal Bronsted acid sites for C-C bond formation and its shape selective pore structure to enable benzene formation. Microwave assisted activation allows localized heating on the active sites while reducing the bulk reaction temperature, which can alter side reactions that produce carbon deposition on this catalyst[4]. In this work, the correlations between catalyst activity and reaction parameters under microwave are investigated to determine the activity-structure relationship and the role of carbon under microwave heating.
Mo/HZSM-5 catalyst with 4% Mo loadings was used in this study. For even distribution of heating within the catalyst bed, a silicon carbide (SiC) powder was physically mixed with the catalyst to improve bed heating and temperature uniformity. The catalyst was thermally activated under CO, followed by MDHA reaction while varying temperature, WHSV and methane concentration via microwave-assisted heating. Different characterization tools were used to study its structure, carbon formation and activity such as BET, TPO, XANES, TGA and XRD techniques.
For all runs under microwave, the catalyst was highly selective to benzene and ethylene as primary reaction products, with little selectivity to toluene or xylene. When Mo-ZSM5 was thermally reacted at 7000C, a lower yield of benzene was observed in comparison to microwave reaction at 5000C. LIII edge XANES showed higher Mo oxide reduction under microwave compared to thermal heating, suggesting faster activation of Mo under microwave. This maybe due to the selective heating of Mo active sites compared to ZSM-5 sites, thus producing higher benzene yields at lower bulk temperature.
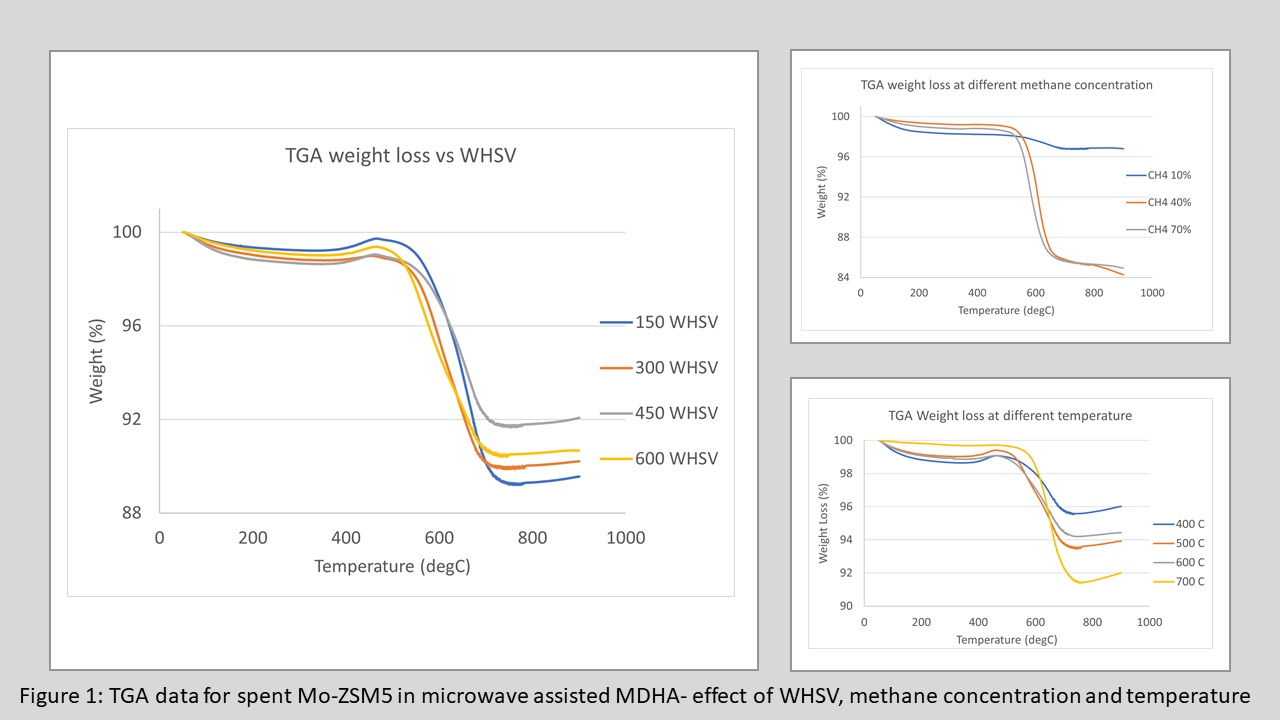
Carbon formed during microwave runs between 400-7000C and analyzed by thermogravimetric analysis (TGA) showed the highest accumulation of carbon at 7000C (figure 1). BET results indicate a reduction in pore volume, which suggest the carbon is depositing in the mouth of the pores. At 7000C in microwave, this results in maximum pore blockage, which restricts the reaction intermediates to access ZSM-5 pores and form aromatics. For temperatures in the range of 500-6000C, the maximum production of benzene can be correlated to polymeric carbon formation observed in TPO. Carbon formation increased with decreasing space velocity(figure 1), as higher gas-solid contact period caused additional carbon deposition in the catalyst. With decreasing space velocity, a transition of carbon formation was observed from polymeric to graphitic region. At 5000C, which is the optimum microwave temperature for higher benzene yield, an increasing amount of carbon was formed for all runs when methane concentration was increased from 10% to 70%(figure 1). Carbon deposition increased and BET surface area decreased when methane concentration increased, indicating higher catalytic activity. This decrease in surface area could be related to higher local temperatures as carbon is an excellent microwave absorber. Deposition of carbon increased with temperature, gas-solid contact period and methane concentration thus enhanced microwave heating and resulted in higher catalytic activity. Compared to microwave reaction at 5000C, thermal reaction at 7000C resulted in lower carbon deposition, which led to deactivation. This indicates the role of the deposited carbon as a possible active site for promoting microwave assisted MDHA. However, a significant temperature gradient exists within the catalyst bed, with core of the catalyst bed reaching a much higher temperature than the surface in microwave, which suggests that in microwave, MDHA can occur at much higher temperature compared to thermal reactions. The study indicates that under traditional heating carbon formation deactivates the active sites, however, under microwaves, the presence of localized carbon plays an important role in coupling microwave energy and leading to localized heating that improves selectivity to benzene.
Keywords: microwave dehydroaromatization, catalyst characterization, microwave deactivation.
References
- Mac Kinnon, M.A., J. Brouwer, and S. Samuelsen, The role of natural gas and its infrastructure in mitigating greenhouse gas emissions, improving regional air quality, and renewable resource integration. Progress in Energy and Combustion Science, 2018. 64: p. 62-92.
- Abedin, M.A., et al., Sulfated hafnia as a support for Mo oxide: A novel catalyst for methane dehydroaromatization. Catalysis Today, 2020. 343: p. 8-17.
- Abedin, M.A., et al., Methane dehydroaromatization using Mo supported on sulfated zirconia catalyst: Effect of promoters. Catalysis Today, 2021. 365: p. 71-79.
- Abdelsayed, V., D. Shekhawat, and R.S. Tempke, Zeolites interactions with microwaves during methane non-oxidative coupling. Catalysis Today, 2021. 365: p. 88-102.