(372d) The Technology Development in Magnesium Production and Separation
AIChE Annual Meeting
2022
2022 Annual Meeting
Separations Division
Poster Session: General Topics on Separations
Tuesday, November 15, 2022 - 3:30pm to 5:00pm
In the late 1800s, an alternative method of producing Mg was introduced using carbothermic reduction. Carbon is pelletized with a source of magnesium oxide, such as dolomite. The following reaction happens when the reactants are heated to around 2,000 K at 1 atm:
MgO + C → Mg + CO (1)
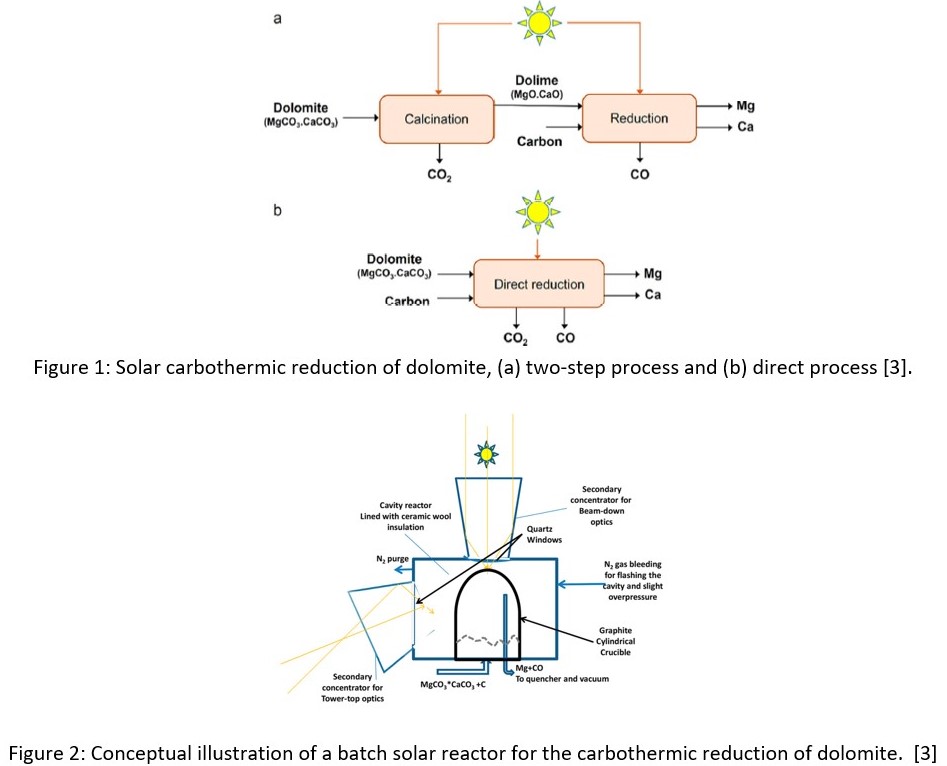
Previously, it was thought that dolomite needed to undergo a calcination step before reduction, as shown in Figure 1 a. Recently the direct reduction of dolomite into magnesium and calcium has been investigated. The results show that the direct method requires less energy and releases a smaller quantity of CO2 [3]. To further decrease the energy needed to produce the magnesium, a solar batch reactor like the one shown in Figure 2 can be used [3].
Carbothermic reduction has shown itself to be a promising alternative to current methods. It significantly decreases the quantity of energy used and the amount of CO2 released by the process. A cradle-to-gate life cycle study found that when using solar reactors to reduce dolomite, it takes only 51.3 Mj, and results in the release of 12.7 kg CO2 per kg of Mg produced [4]. Whereas the Pidgeon process currently used in most magnesium production facilities requires 366 Mj and releases 42 kg of CO2 per kg of Mg produced [4]. Carbothermic reduction’s significant increase in efficiency opens the possibility of once again producing magnesium domestically.
Unfortunately, the product of this method is a mixture of magnesium vapor and carbon monoxide gas, which quickly reverts the magnesium vapor into magnesium oxide once the temperature begins to decrease. Researchers have been attempting to solve this reversion reaction of equation 1 for over 100 years and have developed many methods that lessen but do not solve the problem.
The many attempts to separate magnesium from carbon monoxide can be divided into three general categories [5]. The first of these methods involves rapidly quenching the magnesium gas, resulting in the formation of small magnesium particles directly from the gas state. The second relies on rapidly condensing the magnesium onto a cold surface. Lastly, some processes use a solvent such as another metal or a hydrocarbon that cools and absorbs the magnesium. However, despite over a century of research and the development and subsequent closing of several production plants, no magnesium is currently being commercially produced via the carbothermic reduction route.
It is necessary to investigate further all the product magnesium produced by the previous separation methods. No method has been able to stop the reversion process from producing pure magnesium. An additional sublimation or distillation method has always been needed, resulting in a significant increase in the operating cost. However, there is a method that may be able to produce pure magnesium directly from the product gases. The new process being considered would separate the magnesium vapor from the carbon monoxide before the mixture can cool down from the reaction temperature. The result would be pure magnesium that had been protected from the temperature conditions that lead to oxidation. One possible way of accomplishing this task is using large beds consisting of activated carbon or other molecular sieves. Little work has been done in this area because these separations suffer an extreme loss in efficiency at high temperatures. Fortunately, even with low efficiency, the cost of running such a system would theoretically be less than having an entire distillation stage in the process. Further investigation is required to determine the efficiency of a high-temperature adsorption bed.
References:
- “Metal Magnesium Market Size, Share & Trends Analysis Report By Application (Die Casting, Aluminum Alloys, Titanium Reduction, Iron & Steel Making), By Region, And Segment Forecasts, 2020 – 2027,†Grand View Research, https://www.grandviewresearch.com/industry-analysis/metal-magnesium-market. (Accessed October 5, 2021).
- M. Halmann, A. Frei, and A. Steinfeld, “Magnesium Production by the Pidgeon Process Involving Dolomite Calcination and MgO Silicothermic Reduction: Thermodynamic and Environmental Analyses,†Industrial & Engineering Chemistry Research, vol. 47, no. 7, pp. 2146-2154, Apr. 2008, doi: 10.1021/ie071234v.
- A. Najafabadi, N. Ozalp, M. Epstein, and R. Davis, “Solar Carbothermic Reduction of Dolomite: Direct Method for Production of Magnesium and Calcium,†Industrial & Engineering Chemistry Research, vol. 59, no. 33, pp. 14717-14728, Aug. 2020, doi: 10.1021/acs.iecr.0c02329.
- H. A. Najafabadi, N. Ozalp, M. Epstein, and R. Davis, “Solar Carbothermic Reduction of Dolime as a Promising Option To Produce Magnesium and Calcium,†Industrial & Engineering Chemistry Research, vol. 58, no. 51, pp. 23540-23548, Dec. 2019, doi: 10.1021/acs.iecr.9b04856.
- B.A. Chubukov, S.C. Rowe, A.W. Palumbo, et al., “Investigation of continuous carbothermal reduction of magnesia by magnesium vapor condensation onto a moving bed of solid particles,†Powder Technology, vol 365, pp. 2-11, Apr. 2020, https://doi.org/10.1016/j.powtec.2019.01.067