(356d) Enhancing the Capture Efficiency of Antibody-Antigen Reactions in Sessile Droplets and the Study of Resultant Deposition Patterns
AIChE Annual Meeting
2022
2022 Annual Meeting
Meet the Candidates Poster Sessions
Meet the Industry Candidates Poster Session: Particle Technology Forum
Tuesday, November 15, 2022 - 1:00pm to 3:00pm
Introduction
For immunosensors, the major performance parameters are the capture efficiency and the detection times. Using sessile droplets on functionalized substrates as micro-reactors for antibody-antigen binding reactions reduces the time and the analyte volume required for detection due to the convective currents which cause mixing within the droplet. Since antibody and antigens are charged particles, the capture of antigens by surface functionalized antibodies can be enhanced by tuning the surface charges on them, which in turn can be achieved by tuning the pH of the buffer in which these proteins are dispersed. Colloids (polystyrene microspheres) can be used in these systems to study the velocity profiles and eventually their effect on deposition patterns can be observed. Changes in the zeta potential values of proteins and colloids on changing the buffer pH also affect the particle-particle and particle-substrate DLVO forces and these can be tuned to achieve their homogeneous distribution. While effects of pH and temperature on the transport and surface reaction of these molecules and the influence of pH on deposition patterns have been studied, there are no works previously reported that have studied how pH of the buffer influences the capture efficiency, deposition patterns and the velocity profiles within the droplet in droplet-based systems.
Methods
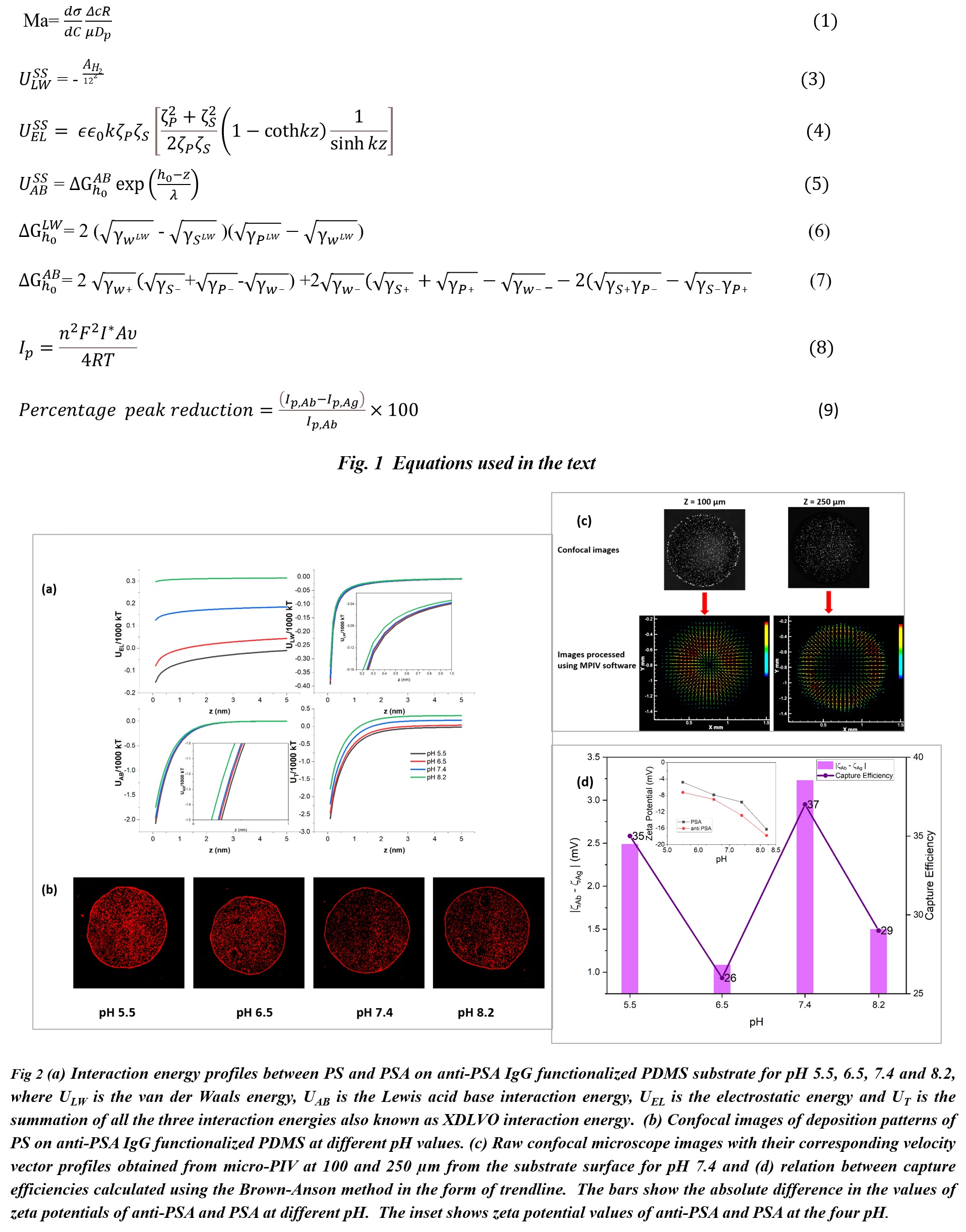
In this work the effect of pH of the buffer on the deposition patterns, the velocity profiles within the sessile droplets containing antigens (PSA) on antibody (anti-PSA IgG) functionalized substrates and the capture efficiency of PSA/anti-PSA in droplet based systems was studied. Polystyrene microspheres have been used in various biological assays for immunodetection due to their non-toxic nature[1,2] and therefore in this study as well these were used as tracer particles for PIV studies and for studying the patterns formed after droplet evaporation and also their effect on the substrate-particle energetics. Micro particle image velocimetry was performed to visualize the internal flow profiles and to obtain the velocity vectors which were found to be resultant of the Marangoni, XDLVO and surface forces present.
The strength of Marangoni convection can be analyzed by the order of the Marangoni number (Ma) which is defined as [3,4] Eq.1 (given in Fig.1). Here, C is the concentration of solute, Δc is the concentration difference of the solute at the droplet edge and center, R is the radius of the droplet and µ is the viscosity of the liquid.
Extended DLVO (XDLVO) force analysis between the particles (PSA and polystyrene microspheres) and substrate (PDMS) was done to obtain potential energy versus distance plots for the different pH values used in this study. The total energy (UT) of interaction can be given as [5]:
UT = ULW + UEL+ UAB (2)
where, UEL is the electrostatic interaction, ULW is the van der Waals interaction and UAB is the Lewis acid-base interaction.
The interaction energies per unit area for each component for between two infinite surfaces are given by the following -
the van der Waals attractive interactions, expressed as[6] Eq.3 and the electric double layer (electrostatic) interactions, expressed as [6] Eq.4. Here, AH is the Hamaker’s constant, k is the reciprocal of Debye length, z is the distance between the substrates, ξS and ξP are the zeta potentials of substrate and particles respectively (mV), ε is the permittivity of the medium and ε is the permittivity of vacuum.
and the Lewis acid base interactions, expressed as[7] Eq. 5. Here ΔGh0LWand ΔGh0AB are the Lifshitz-van der Waals free energy and the acid-base free energy, respectively, between two particles or between the particle and the substrate, and are given by [5] Eq. 6 and 7 respectively. Here, h0 (0.158 nm) is the minimum equilibrium distance between two condensed phases, λ is the characteristic decay length of acid-base interactions in water [7]
Capture efficiency of the PSA/anti-PSA system was calculated by doing cyclic voltammetry (CV) experiments. The surface concentration of the adsorbed surface-active species can be calculated using the plot of peak current (IP) versus scan rate (υ) in agreement with the Brown-Anson model[8] using Eq.8. Here, n is the number of electrons transferred, F is the Faraday’s constant, I* is the surface concentration (mol/cm2), A is the electrode surface area (cm2), R is the gas constant and T is the temperature (K). The capture efficiency can be calculated by the difference in the peak currents before and after surface reaction and is given by Eq. 9. Here, Ip,Ab is the peak current after antibody immobilization and Ip,Ag is the peak current after antibody-antigen binding.
Results and discussion
The micro particle image velocimetry (PIV) analysis showed the presence Marangoni flow due to gradients in the solute concentration (Fig.1(c)). Recirculating radially inward flow was observed which is a characteristic of the Marangoni flow. Marangoni number was found to be larger than the critical Ma for solutal instabilities (Ma>100) [9]. Extended DLVO (XDLVO) force analysis was done for particle-substrate to quantify for the observed deposition patterns (Fig.1 (a) and (b)). The Lewis acid base interactions were strong in all cases and were one to two orders of magnitude higher than EL and LW. The EL and LW interactions varied as the pH of the buffer were changed due to changes in the surface charge of the particles and substrate. To understand the extent of reaction and the effect of changing buffer pH, CV and EIS were conducted. The capture efficiencies were found to be proportional to the difference between the absolute zeta potential values of anti-PSA and PSA and the highest capture efficiency was observed for pH 7.4 (Fig. 1(d)). Electrochemical impedance spectroscopy was done to confirm the capture efficiency measurements and verify the species immobilization.
References:
[1] O.D. Velev, E.W. Kaler, In Situ Assembly of Colloidal Particles into Miniaturized Biosensors, 15 (1999) 0–5.
[2] C. Pichot, T. Delair, A. Elaissari, E. Normale, S. De Lyon, POLYMER COLLOIDS FOR BIOMEDICAL AND PHARMACEUTICAL, (1997) 515–539.
[3] R.G. Larson, Transport and deposition patterns in drying sessile droplets, AIChE J. 60 (2014) 1538–1571. https://doi.org/10.1002/aic.14338.
[4] H. Hu, R.G. Larson, Analysis of the effects of marangoni stresses on the microflow in an evaporating sessile droplet, Langmuir. 21 (2005) 3972–3980. https://doi.org/10.1021/la0475270.
[5] C.J. van Oss, Acid-base interfacial interactions in aqueous media, Colloids Surfaces A Physicochem. Eng. Asp. 78 (1993) 1–49. https://doi.org/10.1016/0927-7757(93)80308-2.
[6] M. Elimelech, J. Gregory, X. Jia, R.A. Williams, Particle Deposition and Aggregation, Butterworth Heinemann publications, Woburn, MA, 1998.
[7] C.J. Van Oss, The Properties of Water and their Role in Colloidal and Biological Systems, 2008. https://doi.org/10.1016/S1573-4285(08)00203-2.
[8] A.P. Brown, F.C. Anson, Cyclic and Differential Pulse Voltammetric Behavior of Reactants Confined to the Electrode Surface, 49 (1977) 1589–1595.
[9] A. Darras, N. Vandewalle, G. Lumay, Transitional bulk-solutal Marangoni instability in sessile drops, Phys. Rev. E. 98 (2018) 1–7. https://doi.org/10.1103/PhysRevE.98.062609.