(319b) Catalytic Conversion of Methane to Hydrogen and Carbon Nanotubes By Microwave Irradiation
AIChE Annual Meeting
2022
2022 Annual Meeting
Fuels and Petrochemicals Division
Fuel Processing for Hydrogen Production
Tuesday, November 15, 2022 - 12:46pm to 1:02pm
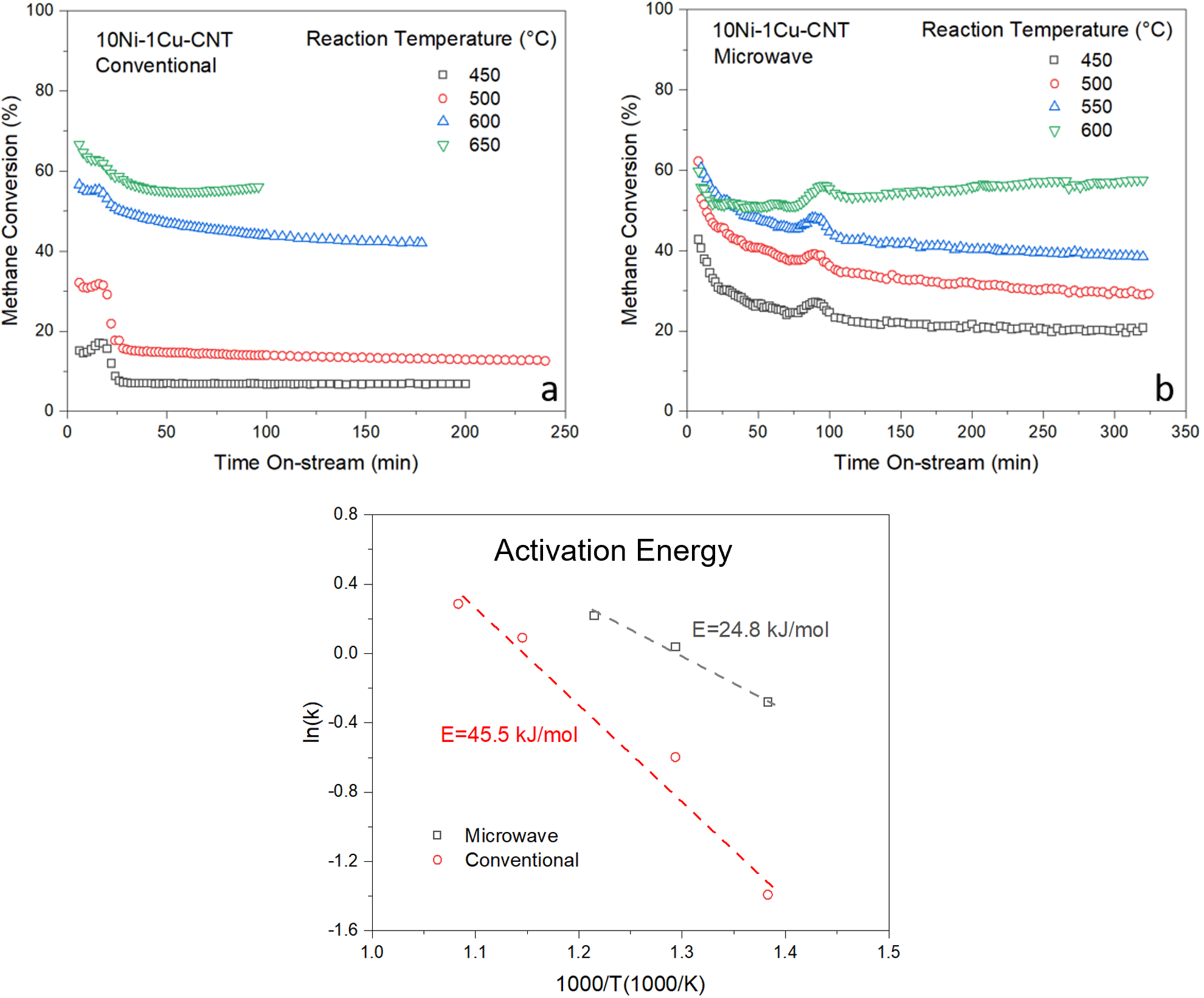
Carbon nanotubes supported Ni-Pd or Ni-Cu bimetallic catalysts were adopted to dehydrogenate methane and produce carbon nanotubes and hydrogen. The catalytic reactions were driven by microwave irradiation only. Both catalysts showed methane conversions over 35% at 550 °C. Ni-Cu displayed a better dehydrogenation performance and a lower cost when compared to Ni-Pd. The TEM and Raman characterizations of spent catalysts have indicated that the solid products were carbon nanotubes. The activation energy of 10Ni-1Cu-CNT was calculated as 24.8 kJ/mol under microwave irradiation, which was much lower than that of conventional heating (45.5 kJ/mol). The results have indicated that 10Ni-1Cu-CNT catalyst could be further scaled up and promoted for simultaneously converting methane to carbon nanotubes and hydrogen under microwave irradiation. Additionally, carbon nanotubes could be sold to reduce the overall cost for hydrogen production. Technoeconomic analysis indicates that the technology has the potential to lead to $ 1/kg H2.