(318a) Robust Separation Process for Producing Pure Glucaric Acid Crystals from Fermentation Broth
AIChE Annual Meeting
2022
2022 Annual Meeting
Forest and Plant Bioproducts Division
Advanced Separations Processes in Bioprocessing and Biomaterials
Tuesday, November 15, 2022 - 1:00pm to 1:15pm
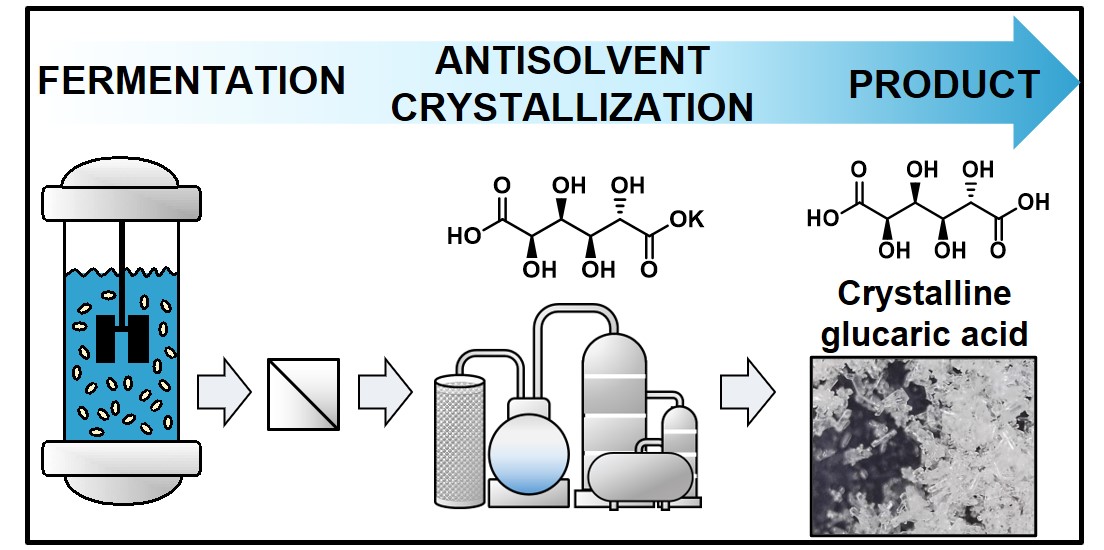
Glucaric acid (GA) is regarded as a top-value added bio-derived compound. GA and its salts have applications in many products including biodegradable detergents, corrosion inhibitors, and polymers precursors or additives. Today, GA is primarily produced via the chemical oxidation of glucose using nitric acid or catalytic oxidation of glucose. These approaches have either an expensive and nonselective or a lower conversion yield and scalability issues. Alternatively, biocatalysis offers high selectivity and conversion yield in mild reaction conditions such that fermentation is promising for producing GA with several green chemistry benefits. However, a major challenge in this bioprocessing is to separate and purify GA from the fermentation broth. That is because GA is dissolved in complex mixtures and, most importantly, GA diacid is readily lactonized into D-glucaro-1,4-lactone, D-glucaro-6,3-lactone, and D-glucaro-1,4:6,3-dilactone in aqueous conditions at low pH. Accordingly, an efficient method for glucaric acid separation, especially its diacid form, is necessary to facilitate its valorization. In this seminar, we present a robust separation process that produces glucaric acid crystals from fermentation broth. The developed process is divided into two-step purification processes: (1) recovering monopotassium glucarate (KGA) from broth and then (2) crystallizing purified GA from KGA through acidification and antisolvent crystallization. Overall, this process was found to separate monopotassium glucarate and glucaric acid with a recovery yield of >99.9 % and 71 % at purities of c.a. 95.6 and 98.3 %, respectively. The overall energy footprint of the developed downstream process will also be discussed based on the post-solvent recovery model.