(217b) Catalytic Conversion of CO2 over La2O3 and CeO2 Supported Catalysts
AIChE Annual Meeting
2022
2022 Annual Meeting
Sustainable Engineering Forum
Novel Approaches to CO2 Utilization II
Monday, November 14, 2022 - 3:45pm to 4:00pm
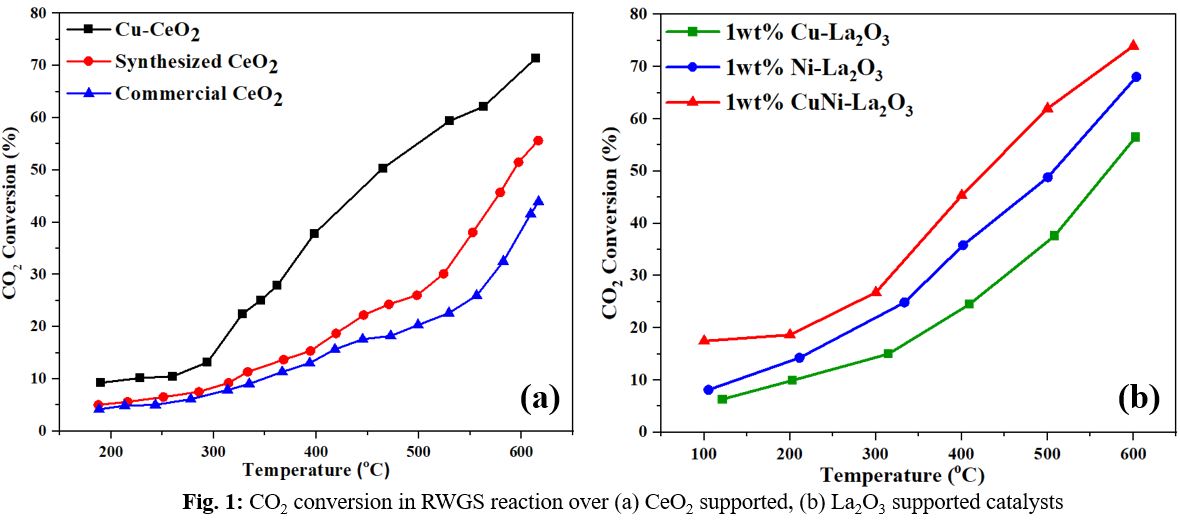
CO2 is a cheap and abundant carbon source, and its chemical conversion into value-added chemicals and fuels reduces CO2 emissions while simultaneously offering significant economic benefits. The reverse water gas shift reaction (RWGS) is one of the most appealing ways of converting CO2 to CO, where after mixing with H2 as syngas, it can be fed into another unit such as the Fischer-Tropsch process to produce fuels and chemicals of choice. The RWGS process is thermodynamically favorable at high temperatures due to its endothermic nature. However, in the operational phase, to save energy and lower capital and operating expenses, the RWGS reaction should be performed at a low temperature. In this condition, it competes with the highly exothermic CO2 methanation reaction, which generates methane and reduces CO yield. As a result, designing selective catalysts for the RWGS reaction that can eventually lead to cost-effective CO2 hydrogenation remains an important and difficult task. Noble metal catalysts have excellent activity and stability in this approach, but their high cost and limited supply make them unsuitable to employ on industrial scales. In order to address this difficulty, several investigations have been conducted to produce catalysts based on transition metals such as Cu and Ni, which are also effective catalysts in the WGS process, and the active catalysts in the WGS reaction are also effective in the RWGS reaction, though under different operating conditions. One of the best choices for producing nanopowders quickly and efficiently is solution combustion synthesis (SCS). In this study, the role of reducible ceria and lanthanum support deposited with 1wt%Cu, 1wt%Ni, and 1wt% CuNi (Cu=0.5%, Ni=0.5%) active metals using SCS was investigated, and their performance in the RWGS reaction using a tubular packed bed reactor in the temperature range of 100–600°C was evaluated. The results reveal that increasing the temperature improves CO2 conversion, with a maximum CO2 conversion of 70% at 600°C and a steady time on stream (TOS) performance for 1200 min with a negligible carbon deposition of (< 0.05%) after the stability. The high catalyst activity is due to the perfect synergistic interaction amongst the active species via Ce3+-oxygen vacancy-Cu0. The Cu-CeO2 catalyst was also found to have 100% selectivity for CO and no CH4 was detected in the outlet stream. In case of lanthanum supported catalysts, the maximum total CO2 conversion were 57%, 68%, and 74% for Cu-La2O3, CuNi-La2O3, and Ni-La2O3, respectively, at 600°C and excellent stability over a 1440-minute TOS with a carbon deposition rate of less than 3wt% for all three samples. However, only the 1wt% Cu-La2O3 catalyst showed a 100% CO selectivity at all temperatures tested. To understand the morphological properties of the catalysts, as well as the effect of the reaction on the catalysts' surface, a variety of analyses such as XRD, TEM, SEM-EDX, and TPR were conducted on both fresh and used catalysts. The results may open new frontiers for the use of reducible oxides as supports in RWGS and other hydrogenation processes.