(20d) Fluid Viscosity Enhances Breast Cancer Metastasis through Coordinated Activity between Volume-Regulatory and Mechanosensitive Ion Channels
AIChE Annual Meeting
2022
2022 Annual Meeting
Food, Pharmaceutical & Bioengineering Division
Cell and Tissue Engineering: Mechanical Cues and Cell Behavior
Sunday, November 13, 2022 - 4:24pm to 4:42pm
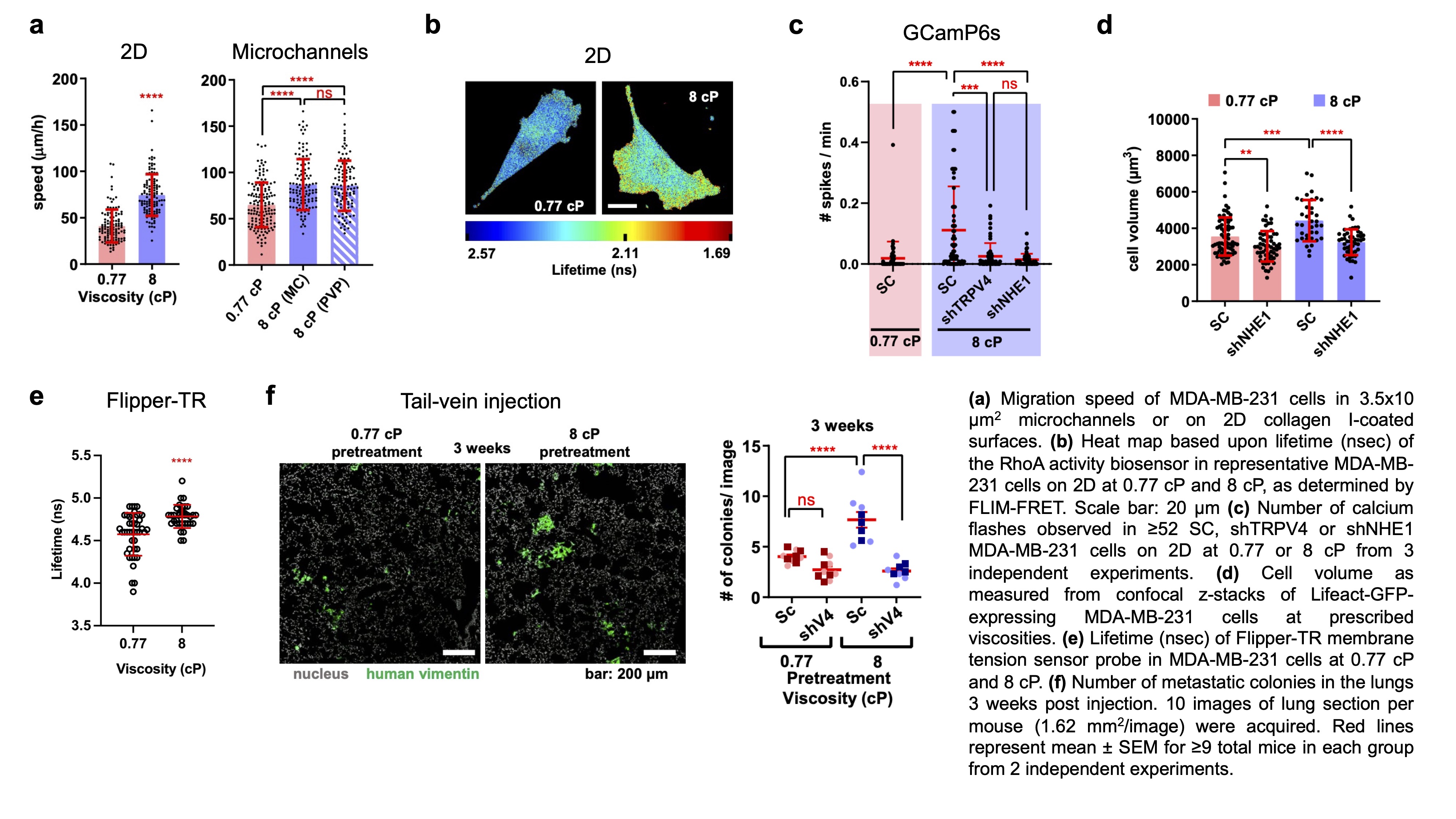
To investigate the effect of fluid viscosity on cells, we designed a microfluidic device with prescribed dimensions that mimics the confining architecture of tissue microenvironments (3.5 x 10 μm2). We found that elevated fluid viscosity (8 cP) counterintuitively increased the motility of MDA-MB-231 breast cancer cells in confinement and on 2D surfaces (Fig. 1a). Live-cell imaging of myosin IIA-GFP followed by immunofluorescence staining of phosphorylated (Ser19) myosin light chain 2 (pMLC) revealed increased myosin II activity at the leading edge of cells migrating at 8 cP. MIIA activity is regulated by RhoA and Rho-associated protein kinase (ROCK). Therefore, we performed FLIM/FRET imaging of the RhoA2G biosensor and found significantly elevated RhoA activity in cells exposed to 8 cP (Fig. 1b). Myosin IIA (MIIA), but not myosin IIB, knockdown, reduced cell motility at 8 cP down to basal levels. Similarly, Y27632 (10 µM), which inhibits ROCK, or blebbistatin (50 µM), which inhibits myosin ATPase activity, abrogated viscosity-induced increases in cell migration.
Actomyosin contractility is regulated by intracellular Ca2+ levels via Ca2+ channels in the endoplasmic reticulum and plasma membrane. For this reason, we performed whole-cell patch clamp recordings and live-cell imaging of the genetically encoded fluorescent Ca2+ indicator GCaMP6s to quantify Ca2+ influx. We found elevated calcium activity in breast cancer cells exposed to 8 cP (Fig. 1c). Accordingly, both cell impermeable (BAPTA) and permeable (BAPTA AM) calcium chelating agents abolished the viscosity-induced enhancement of cell migration. Given that fluid viscosity applies mechanical forces to the cell exterior, we hypothesized that mechanosensitive ion channels in the plasma membrane facilitate elevated Ca2+ influx in response to viscosity. Indeed, Ca2+ activity in response to 8 cP was abolished in cells with TRPV4 knockdown (Fig. 1c), or upon treatment with the TRPV4 inhibitor HC067047, preventing an increase in cell migration speed.
Changes in cell membrane tension can lead to membrane channel activation. Interestingly, we observed that cells exposed to elevated viscosity experienced a rapid increase in cell volume which was dependent on the expression of Na+/H+ exchanger 1 (NHE1) (Fig. 1d). FLIM imaging of the membrane tension probe Flipper-TR revealed significantly elevated plasma membrane tension in cells exposed to 8 cP (Fig. 1e). Consequently, NHE1 knockdown abrogated viscosity-induced increases in Ca2+ activity (Fig. 1c) and migration speed. To determine the physiological significance of fluid viscosity sensing through the coordinated activity of NHE1 and TRPV4, we performed tail-vein injection experiments in mice with pre-conditioned (8 cP) or naïve (0.77 cP) breast cancer cells and quantified lung metastatic burden. Pre-treatment with 8 cP media significantly increased metastatic burden after 3 weeks. In contrast, TRPV4 depletion reduced metastatic burden in both naïve and pre-conditioned cells after 3 weeks (Fig. 1f).
Cumulatively, our data reveal that extracellular viscosity is a novel physical cue, which regulates migration and metastasis in vivo. In particular, we highlight the cooperation of volume-regulatory and mechanosensitive ion channels in this process. This finding is especially important in the field of cell biology as most in vitro functional assays are currently performed in medium with a viscosity close to water (0.7 cP), which is far below the viscosity of interstitial fluid (~2-3 cP) and whole blood (4.5-6 cP).