(173ae) CuNi Catalyst and Its Structural Evolution for Electrochemical Reduction of CO2
AIChE Annual Meeting
2022
2022 Annual Meeting
Catalysis and Reaction Engineering Division
Poster Session: Catalysis and Reaction Engineering (CRE) Division
Wednesday, November 16, 2022 - 3:30pm to 5:00pm
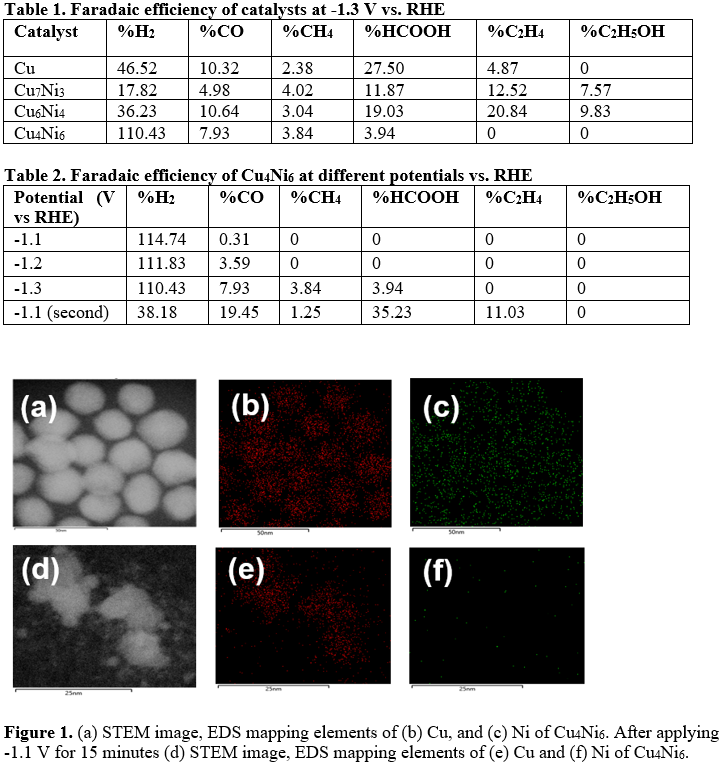
With the rapid increase of CO2 emission and energy demand, the electrochemical reduction of CO2 has attracted tremendous research efforts as a promising strategy to mitigate environmental issues and store renewable energy. Copper (Cu), as an inexpensive and earth-abundant metal electrocatalyst, converts CO2 into valuable chemicals and fuels, especially for C2+ products. This unique property of Cu is due to its appropriate adsorption of *CO intermediates and the subsequent C-C coupling. However, Cu catalysts are impeded by poor product selectivity and dynamically evolving structural motifs. In this work, we alloyed Cu with nickel (Ni) to promote C-C coupling and investigate the structural evolution during CO2RR. We optimized the ratio between Cu and Ni for ethylene and ethanol production. Also, we observed the structural evolution of CuNi nanoparticles using identical location STEM and STEM-EDS.