(170b) Acute Exposure to e-Cigarette Vapor Promotes Neutrophil-Platelet Aggregation in Murine Pulmonary Microvasculature
AIChE Annual Meeting
2022
2022 Annual Meeting
Materials Engineering and Sciences Division
Poster Session: Materials Engineering & Sciences (08E - Electronic and Photonic Materials)
Monday, November 14, 2022 - 3:30pm to 5:00pm
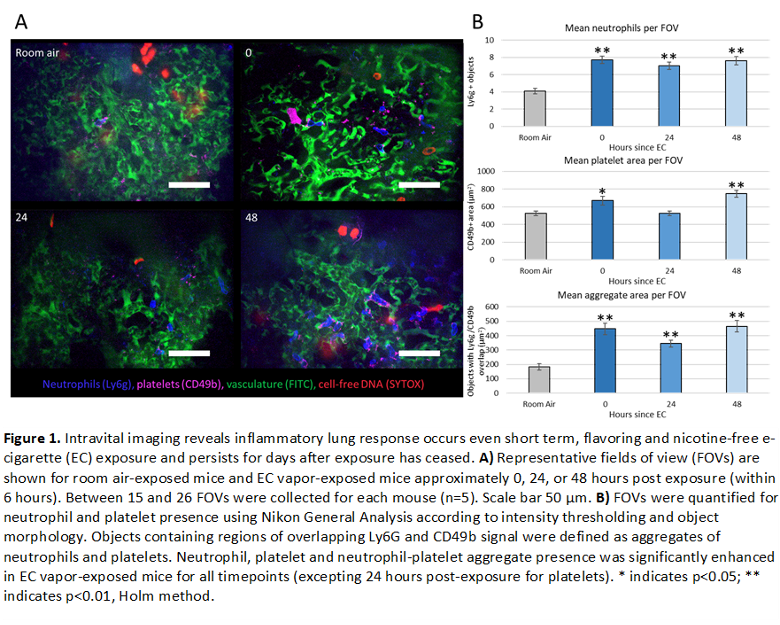
Female BALB/c mice underwent 3 consecutive hours per day of ECV exposure (1 puff/min at 30 watts, 50% vegetable glycerin (VG)/ 50% propylene glycol (PG)), for 3 days. Control mice were exposed only to room air. Analytes were collected from mice at 3 different time points following exposure: immediately after the final exposure (t=0hrs), one day after the final exposure, (t=24hrs), and 2 days after the final exposure (t=48hrs). Intravital imaging of the pulmonary lung vasculature (n=5 mice per group) was performed at each time point. Fluorescent labels were introduced via retro-orbital injection of: FITC dextran (vasculature), Pacific Blue Ly6G (neutrophils), AlexaFluor647 CD49b (platelets), and SYTOX orange (DNA) (Figure 1A). Neutrophil and platelet number was assessed by measuring count and overall area, respectively. NPAs were defined as any object containing both CD49b+ and Ly6g+ signal. Results indicated a significant increase of neutrophils and NPAs in all ECV treated mice. Interestingly, an increase in platelets was observed only in mice either immediately after or 48 hours after ECV exposure, suggesting two different mechanisms for acute and persistent platelet stimulation following ECV exposure (Figure 1B).
Whole blood and bronchoalveolar lavage fluid (BALF) were collected from an additional subset of ECV-exposed and control mice (n=5 per group) at each timepoint. Cell populations in whole blood and the cell pellet from collected BALF were characterized using a ProCyte Dx Hematology Analyzer. Lungs were also collected from n=3 mice, fixed in formalin, sectioned, and stained with hematoxylin and eosin (H&E). Cell counts from ProCyte analysis were not significantly altered in BALF or plasma for any ECV-exposed groups compared to control; however, a non-significant increase in lymphocyte presence in BALF was noted t=24hrs after exposure, possibly indicating lung inflammation. Although an increase in neutrophils was not observed via ProCyte in peripheral whole blood, the increased presence of neutrophils observed in intravital imaging suggests this is a highly localized response. Lastly, plasma and BALF were analyzed to assess the presence of cell free DNA, oxidative stress, myeloperoxidase- a component of NETs, and inflammatory cytokines. Results of these assays are pending.
Overall, our preliminary data indicate a complex immune response occurs upon acute exposure to ECV that persists in the pulmonary vasculature for at least 3 days after cessation of exposure. This work will not only serve to better inform the public of the risks of e-cigarette use, but will also be beneficial to clinicians in characterizing newly emergent pathologies resulting from e-cigarette usage.