(101f) Upgrading Biomass Using Cooperative Interactions in Aminosilica Materials for Aldol Condensation: Discovering Different Types of Catalytic Sites in Aminosilica Materials
AIChE Annual Meeting
2022
2022 Annual Meeting
Catalysis and Reaction Engineering Division
Microporous and Mesoporous Materials III: Structure
Monday, November 14, 2022 - 2:00pm to 2:18pm
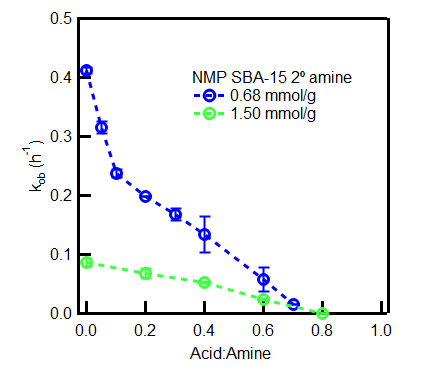
We demonstrate that multiple types of sites exist in aminosilicas through using site quantification. For standard aminosilica material synthesis (REG), we show that only 30% of the overall sites are active. Through reducing the micropore volume of the bare support (NMP), we produce an aminosilica that has an increased fraction of active sites of 50%. This has important implications for the aldol condensation of furfural with acetone. We observe that the NMP materials had more than double the catalytic activity than the REG materials. Importantly, the difference can be attributed to the different fraction of active sites.
As multiple sites exist, we sought to investigate previous synthesis-structure-function behavior, including the effect of surface density. Interestingly, we discover that there are three types of active sites. At high surface densities, each active amine has low activity since the surface density since the high density disrupts cooperative amine-silanol interactions. At moderate densities, we identify that some amines are highly active and contribute significantly to the overall observed catalytic activity. Through tuning the surface density, we increase the fraction of highly active sites. Ongoing experiments will elucidate differences in the structure of amines.