(758a) Condition-Dependent Pd Speciation and NO Adsorption in Pd/Zeolites
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Microporous and Mesoporous Materials III: Metal Chemistry
Thursday, November 19, 2020 - 8:00am to 8:15am
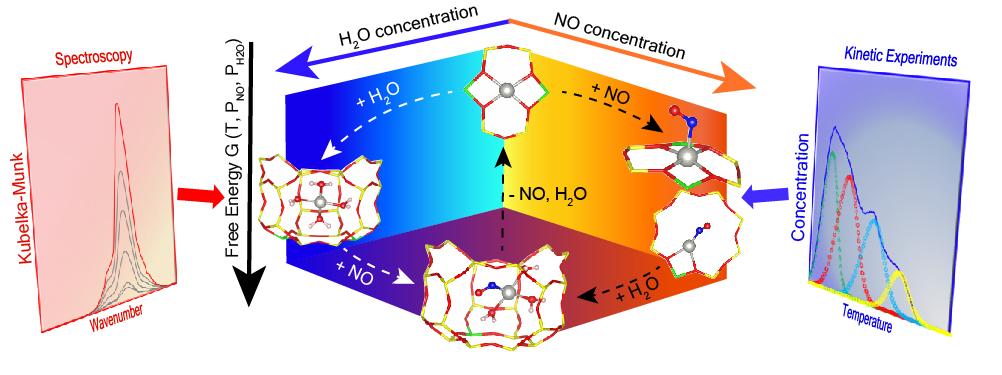
Metal exchanged zeolites and their interaction with other compounds typify a behavior distinct from both the zeolites’ heterogeneous properties and the metals’ homogeneous inorganic chemistry, which is still not well-understood. This poses a challenge in the optimal design and synthesis of these materials for applications such as emissions control where the underlying association of the exchanged metal cations with gases like H2O, NO, etc. is critical. In this study, we examine Pd cations exchanged in SSZ-13 zeolites and their complexing with H2O and NO at a molecular level, employing experimental and computational modeling techniques. Density functional theory (DFT) analyses aided by spectroscopic characterization show that exchanged Pd ions preferentially charge-compensate two Al (2Al) sites in the six-membered ring of SSZ-13 as PdII, akin to similarly sized CuII and CoII analogues. We further probe the reaction environment-dependent Pd coordination chemistry and reactivity using in-situ spectroscopy and kinetic studies, along with ab initio molecular dynamics, coupled-cluster calculations, and first-principles based thermodynamic analyses to arrive at probable molecular structures. Computational modeling and experimental results establish solvation and enhanced mobility of Pd ions at 2Al sites by H2O below 573 K, forming four-fold coordinated square planar complexes as observed in homogeneous Pd compounds, detached from the zeolite framework. Addition of NO facilitates transformation from 2Al to 1Al sites, resulting in H2O-solvated Pd-nitrosyl complexes in the form of [PdII(NO-)(H2O)3]+ that interact only electrostatically with the zeolite framework. These complexes exhibit higher desorption temperatures than their dehydrated counterparts, while also inhibiting competitive CO adsorption. Moreover, in-situ infrared spectra of zeolites with different topologies, such as Pd/BEA and Pd/ZSM-5, demonstrate formation of similar H2O-solvated Pd-nitrosyl complexes, reinforcing the role of H2O in solvation and homogenization of Pd ions in the presence of NO across zeolites of varying frameworks.