(711f) Flow Chemistry-Enabled Investigations of Switchable Hydrophilicity Solvents
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Sustainable Engineering Forum
Green Chemistry and Engineering-I
Tuesday, November 17, 2020 - 9:15am to 9:30am
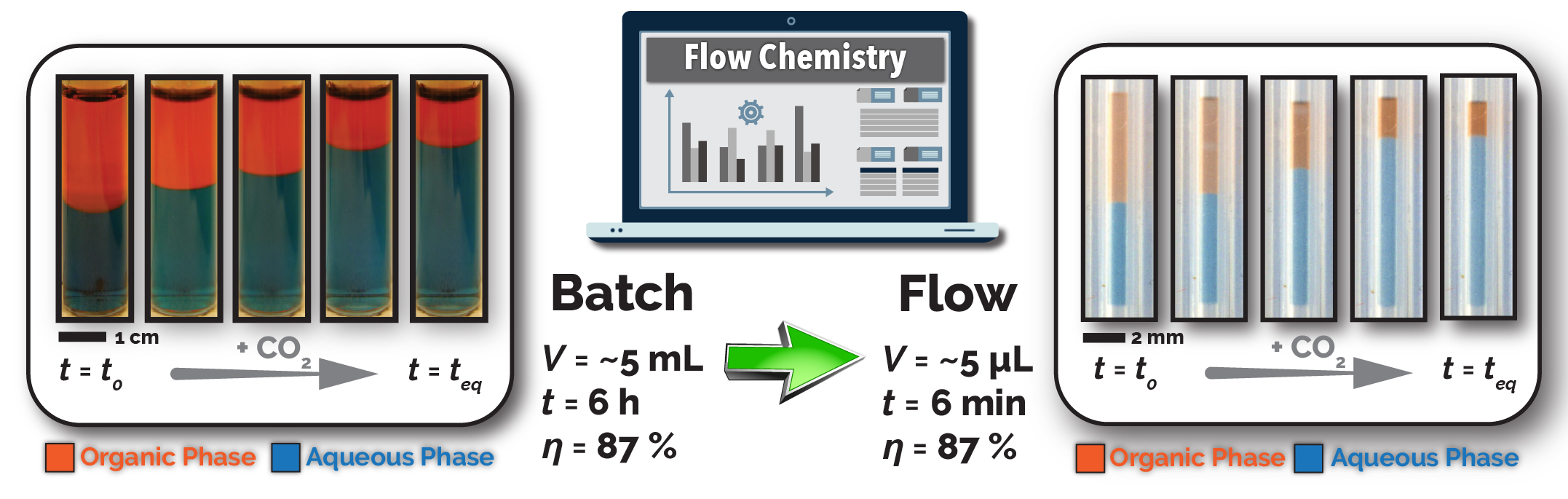
Despite the groundbreaking advancements in the discovery and characterization of SHSs over the last decade, the time-, material-, and labor-intensive nature of conventional batch (flask-based) reactors have hindered further exploration of the broad parameter space of SHSs. Conventional batch reactors have significant mass transfer limitations for conducting gas-liquid reactions due to their poorly defined interfacial area (e.g., bubble column provides 50-600 m2/m3). Thus, screening of an SHS-based LLE process using a batch reactor might take up to10 h/experiment, further resulting in a limited available chemical database for the design of next-generation SHSs. Furthermore, batch screening methods require 10-1000 mL of chemical consumption for each SHS characterization experiment, which leads to significant waste generation and high cost for parameter space exploration of SHSs. Thus, lack of comprehensive understanding of thermodynamic characteristics of CO2-triggered hydrophilicity switching of SHSs, mainly due to the above-mentioned drawbacks of conventional batch processes, has negatively impacted the industrial adoption of this exciting class of green solvents.
In this work, we developed and utilized an automated microscale flow chemistry platform utilizing a membrane-based flow reactor for accelerated studies of both continuous (e.g., CO2 pressure, reaction time, SHS concentration, and flow velocity) and discrete (e.g., the chemical structure of SHS) process parameters associated with CO2-mediated SHS recovery process. Utilizing a highly CO2-permeable membrane in a tube-in-tube configuration, offering a significantly higher interfacial area of 4900 m2/m3 than conventional batch reactors, we systematically studied CO2-mediated SHS extraction efficiency and kinetics, in situ, as fast as 4 min/experiment (~100 times faster than batch reactor), while utilizing only 4-8 μL of SHS mixture (~2500 times less chemical consumption than batch reactor) for each experimental condition. The single-droplet flow reactor provided a time- and material-efficient approach for comprehensive fundamental and applied studies of the extraction efficiency and kinetics of SHS-based LLE processes, providing accelerated access to the optimized process conditions. Next, the tube-in-tube flow reactor was reconfigured from the single-droplet screening mode to the continuous flow configuration to continuously recover the SHS under the optimized conditions identified by the single-droplet process screening flow reactor.2 Since the single-droplet and continuous flow reactor configurations share the same intensified mass transfer characteristics, the optimized conditions identified by the screening flow reactor are directly transferrable to the continuous flow reactor. The proposed research will provide a promising solvent utilization technique with valuable insight and robust guidelines for green and sustainable chemical processing.
Reference.
(1) Jessop, P. G.; Mercer, S. M.; Heldebrant, D. J. CO2-Triggered Switchable Solvents, Surfactants, and Other Materials. Energy Environ. Sci. 2012, 5 (6), 7240–7253. https://doi.org/10.1039/C2EE02912J.
(2) Han, S.; Raghuvanshi, K.; Abolhasani, M. Accelerated Material-Efficient Investigation of Switchable Hydrophilicity Solvents for Energy-Efficient Solvent Recovery. ACS Sustainable Chem. Eng. 2020, 8 (8), 3347–3356. https://doi.org/10.1021/acssuschemeng.9b07304.