(677e) Influence of Active Site Density and Arrangement on the Kinetics of NOx Selective Catalytic Reduction with NH3 over Cu-CHA Zeolites
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Fundamentals of Catalysis and Surface Science III: Solvent Effects in Microporous Materials
Thursday, November 19, 2020 - 9:00am to 9:15am
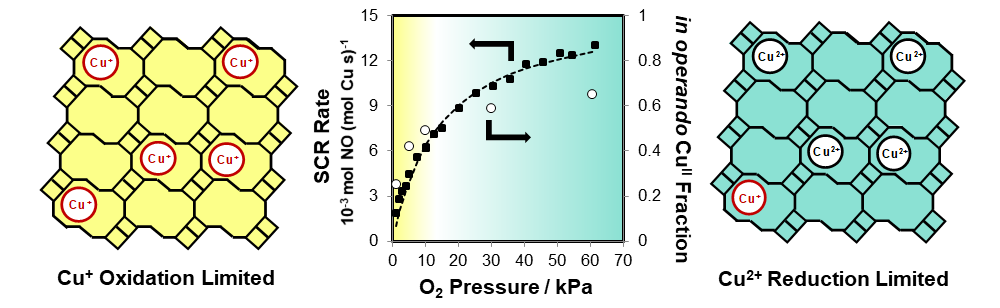
Here, SCR rates are measured over a wide range of O2 pressures, which allows extracting rate constants for oxidation and reduction steps in low and high O2 pressure regimes, respectively (Figure 1). Most abundant reactive intermediates are monitored in operando by X-ray absorption spectroscopy (XAS). On Cu-CHA zeolites of fixed Al content (Si/Al = 15), oxidation rate constants (per Cu)increase monotonically with Cu density, consistent with a dual-site oxidation mechanism. Reduction rate constantsshow a weaker positive dependence on Cu density, predominantly reflecting changes in the fraction of Cu sites that can pair and thus mediate SCR turnovers. These conclusions are consistent with transient XAS measurements showing that increasing Cu density does not systematically influence reduction rate constants, but increases the fraction of Cu(I) that can pair during O2-assisted oxidation. These quantitative kinetic and active site descriptors are measured on CHA zeolites with a range of Cu densities and framework Al densities and arrangements, revealing how the mobility of electrostatically tethered Cu ions impacts both the fraction of sites that catalyze NOx-SCR, and their turnover frequencies.