(651h) Framework for Economic Evaluation of Solvent Recovery Options
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Computing and Systems Technology Division
Process Design in Energy and Sustainability II
Wednesday, November 18, 2020 - 9:45am to 10:00am
Pfizer and Rowan University had carried out an investigation to recover and purify isopropanol (IPA) from the celecoxib process waste stream. The celecoxib process produces the API for an arthritis pain medicine known as Celebrex® [10]. The waste stream following the final purification stage in the pharmaceutical process contains a significant amount of recoverable IPA. However, the results of laboratory-scale distillation and extraction conducted at the plant site failed to reach the purity requirement.
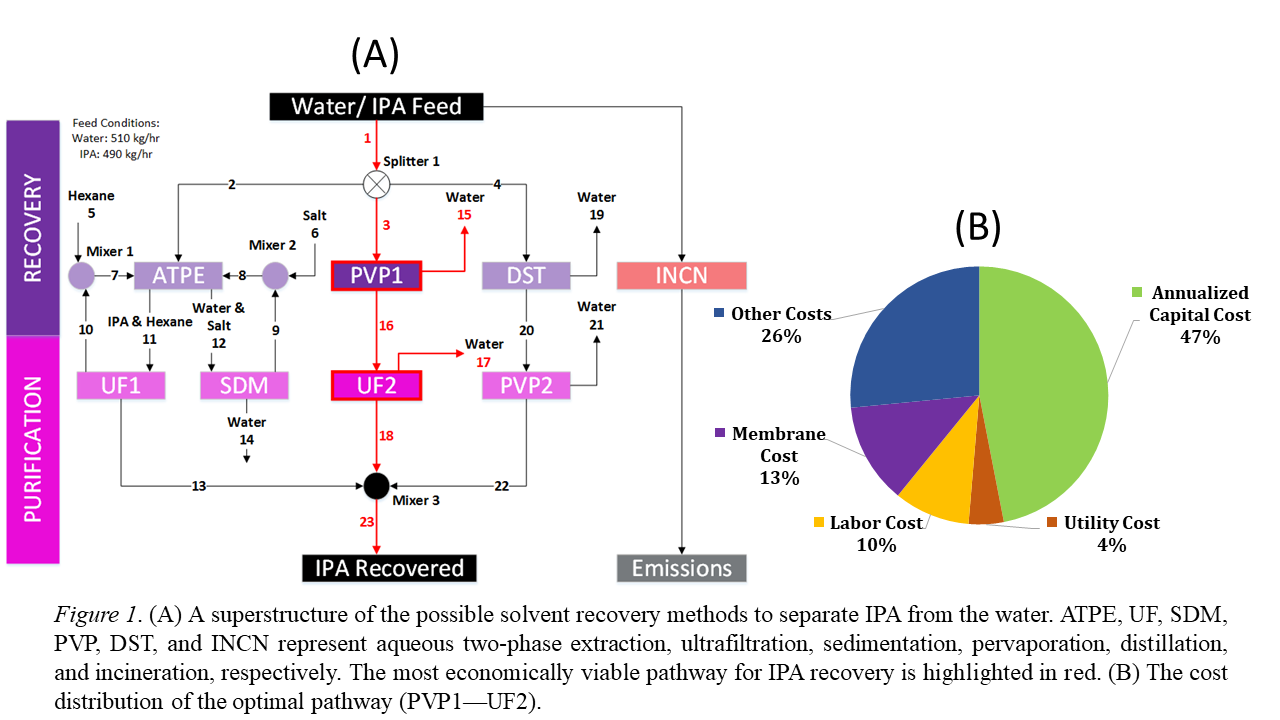
In Figure 1, we developed the case-specific superstructure as a starting point for analyzing process efficiency, cost, and environmental impact of a solvent recovery pathway [11]. This superstructure includes a systematic representation of all the relevant technologies and flow streams in the IPA recovery paths. Mixed-integer non-linear programming (MINLP) problem was formulated and solved using the General Algebraic Modeling Systems (GAMS) with Branch-And-Reduce Optimization Navigator (BARON) as the solver. The optimized IPA recovery pathway from GAMS required the selection of pervaporation (PVP1) and ultrafiltration (UF2). Using a basis of 1000 kg/hr waste feed, this pathway will require a $524,000 annualized cost of operation and $0.14/kg solvent recovered. The continuous incineration of this waste stream requires an annualized cost of $8.1 million, which equates to $2/kg of solvent processed. All solvent recovery pathways are more economically viable than incineration because materials are being recovered and reused within the process.
We are in the process of solving additional case studies from other industrial sectors that will include economic evaluation, metrics from life cycle analysis with considerations for environmental impact associated with each recovery pathway. These case studies will be used to refine our existing solvent recovery framework, which can be translated into a computational tool that can optimize solvent recovery, reuse, and recycling in any solvent consuming industrial process.
References
[1] C. S. Slater, M. J. Savelski, W. A. Carole, and D. J. C. Constable, “Solvent Use and Waste Issues,†in Green Chemistry in the Pharmaceutical Industry, P. J. Dunn, A. S. Wells, and M. T. Williams, Eds. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 2010, pp. 49–82.
[2] M. Tyrer, “Municipal solid waste incinerator (MSWI) concrete,†in Eco-Efficient Concrete, Elsevier, 2013, pp. 273–310.
[3] O. US EPA, “Global Mitigation of Non-CO2 Greenhouse Gases: Solvents,†US EPA, 03-Feb-2016. https://www.epa.gov/global-mitigation-non-co2-greenhouse-gases/global-mi... (accessed Mar. 03, 2019).
[4] E. Heinzle et al., “Ecological and Economic Objective Functions for Screening in Integrated Development of Fine Chemical Processes. 1. Flexible and Expandable Framework Using Indices,†Industrial & Engineering Chemistry Research, vol. 37, no. 8, pp. 3395–3407, Aug. 1998, doi: 10.1021/ie9708539.
[5] H. Chen and D. R. Shonnard, “Systematic Framework for Environmentally Conscious Chemical Process Design: Early and Detailed Design Stages,†Industrial & Engineering Chemistry Research, vol. 43, no. 2, pp. 535–552, Jan. 2004, doi: 10.1021/ie0304356.
[6] C. Jiménez-González, D. J. Constable, A. D. Curzons, and V. L. Cunningham, “Developing GSK’s green technology guidance: methodology for case-scenario comparison of technologies,†Clean Technologies and Environmental Policy, vol. 4, no. 1, pp. 44–53, Jul. 2002, doi: 10.1007/s10098-001-0134-7.
[7] K. A. Hossain, F. I. Khan, and K. Hawboldt, “Sustainable development of process facilities: State-of-the-art review of pollution prevention frameworks,†Journal of Hazardous Materials, vol. 150, no. 1, pp. 4–20, Jan. 2008, doi: 10.1016/j.jhazmat.2007.08.062.
[8] V. H. Hoffmann, K. Hungerbühler, and G. J. McRae, “Multiobjective Screening and Evaluation of Chemical Process Technologies,†Industrial & Engineering Chemistry Research, vol. 40, no. 21, pp. 4513–4524, Oct. 2001, doi: 10.1021/ie001080i.
[9] E. J. Cavanagh, “A new software tool to environmentally and economically evaluate solvent recovery in the pharmaceutical industry,†2014.
[10] C. S. Slater, M. Savelski, G. Hounsell, D. Pilipauskas, and F. Urbanski, “Green design alternatives for isopropanol recovery in the celecoxib process,†Clean Technologies and Environmental Policy, vol. 14, no. 4, pp. 687–698, Aug. 2012, doi: 10.1007/s10098-011-0433-6.
[11] J. Chea, A. Lehr, J. Stengel, M. J. Savelski, C. S. Slater, and K. Yenkie, “Evaluation of Solvent Recovery Options for Economic Feasibility through a Superstructure-Based Optimization Framework,†Industrial & Engineering Chemistry Research, Mar. 2020, doi: 10.1021/acs.iecr.9b06725.