(644c) Development of High-Solids, Quick-Set Pharmaceutical Tablet Coatings.
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Pharmaceutical Discovery, Development and Manufacturing Forum
Novel Excipients in Formulation Design and Drug Delivery
Thursday, November 19, 2020 - 8:30am to 8:45am
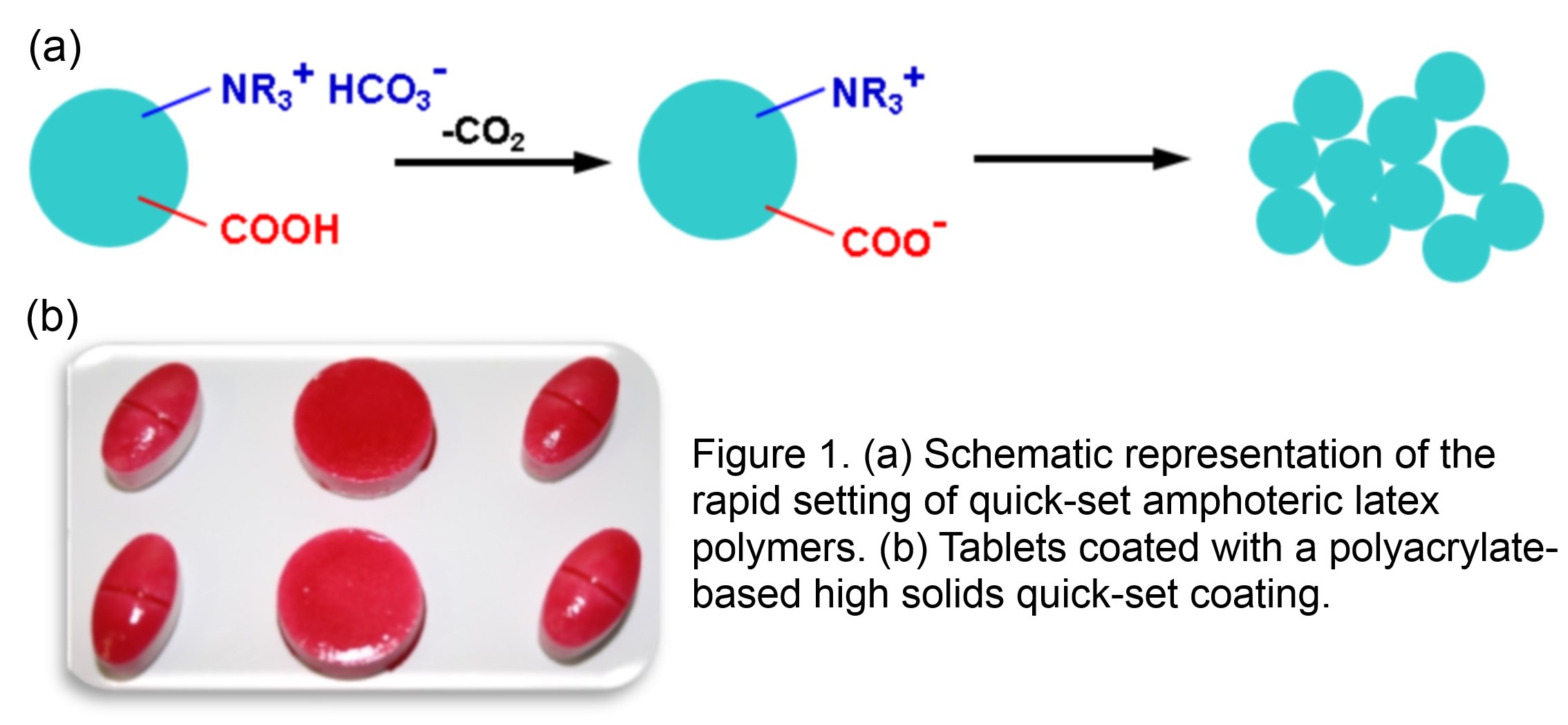
This study2 focused on the evaluation of amphoteric polyacrylate latex-based film forming materials for tablet coatings. A series of amphoteric latexes was prepared from monomers already used in the synthesis of FDA-approved polyacrylate polymers. Polymer composition was systematically varied in order to understand the effects on polymer film formation temperature, setting efficiency as a function of pH, and film properties such as tack, puncture, gloss, optical clarity, water permeability, etc.
Latex films were evaluated using pharmaceutical test methods, and showed equal or superior performance compared to commercially-used cellulosic and poly(vinyl alcohol) coatings. The quick-set efficiency of these latexes was evaluated based on their aggregation behaviors in a series of buffers with pH ranging from 5 to 10. The effect of composition variables on the setting efficiency of latexes was studied. Several performance characteristics for tablet coatings have been measured, including high solids (~40 wt. %), good optical properties (clarity > 90%, transmission > 93%, and haze < 6%), no tackiness, and low permeability (water vapor permeability < 2 x 10-7 g / Pa s m). Mechanical properties of films made from amphoteric latexes are similar to those of PVOH films, but they are softer and more elastic than HPMC films.
We have demonstrated that latexes with a balance of quick-setting efficiency and tunable coating properties can be prepared. Amphoteric latex-based tablet coatings can be performance- and cost-competitive to HPMC- and PVOH-based coatings, easier to apply from a high solids formulation, and their composition can be easily adjusted to provide coatings with a desirable balance of properties. The polyacrylate latex films exhibited excellent mechanical and optical properties, high transmission, high clarity, and low haze. The water vapor permeability of latex films was lower than that of current commercial HPMC and PVOH films. These results suggest that amphoteric latexes can be used to prepare tablet coatings with good appearance and desirable performance. Typical coated tablets are shown in Figure 1(b).
References: 1. (a) Schmidt, D.; Mussell, R.; Rose, G. Coat. Technol. 2003, 75, 59-64. (b) Rose, G. et al. Langmuir 2005, 21, 1192-1200. 2. Ladika, M.; Kalantar, T.; Shao, H.; Dean, S.; Harris, K.; Sheskey, P.; Coppens, K.; Balwinski, K.; Holbrook, D. Appl. Polym. Sci. 2014, 131(7), 539-550.