(602h) Scaling-up of Magnetite Nanoparticles Production for Drug Delivery Applications Via Static Mixers: Role of Turbulent Reacting Flow
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Particle Technology Forum
Particle Technology in Product Design
Friday, November 20, 2020 - 9:30am to 9:45am
Here, we propose to tackle this major issue by maintaining proper mixing conditions via multiphase turbulent reacting flow during the synthesis of magnetite. The first step for the scaling-up of the co-precipitation method was to run the synthesis at the bench scale. In this case, the synthesis proceeded in a semi-batch mode of operation in a 3L stainless steel reactor equipped with a Rushton impeller, and a peristaltic pump to control the addition of the iron chlorides to the reaction mixture. The reaction started by adding the iron (II) and (III) chlorides to a sodium hydroxide solution (5M) at a rate of 10 mL/min while maintaining vigorous agitation at 600 RPM. The temperature was maintained at 90°C throughout the course of the reaction. The obtained material showed a wide size distribution that peaked at around 1 um, which fails to comply with the requirement for the intended application in drug delivery. According to our observations, this larger distribution appears to be related to poor mixing conditions where shear stress was insufficient. We propose to address this issue by locating a static mixer followed by a venturi system and a second static mixer on a recycling pipe that is constantly taking the reaction mixture and returning it after passing by the in-line mixing device (Figure 1). As a result, we hypothesize that this assembly should suffice to produce a turbulent reacting flow regime where growth and nucleation lead to the material with the required size. The proposed system was first evaluated in silico with the aid of COMSOL Multiphysics V.5.3. We considered two different types of mixers, one with a corrugated topology (Figure 2A) and second with a helicoidal one (Figure 2B). In COMSOL we implemented the chemical reactions and the transport of diluted species modules, which allowed us to estimate shear stress, input flows, pressure drop, and the proper location on the pipe. The computational domain of the complete assembly using the helicoidal mixer is shown in Figure 2C, which was discretized via about 600,000 free tetrahedral elements with a refinement in the edges prior to running the simulation to achieve convergence.
As the simulations indicated high-stress levels for the corrugated plates static mixer we, therefore, proceeded to manufacture such assembly. Experimental tests at the bench-scale confirmed a sharper particle size distribution with an average of about 120 nm. As opposed to the initial synthesis, the obtained material complies with the requirements of our application in drug delivery. Further tests at the pilot plant scale will be needed to adjust geometrical parameters of the mixers for evaluating yields and particle size distributions.
References:
1. Nano – A comprehensive nanotechnology database by Nature Research. Available at: https://nano.nature.com/?gclid=CjwKCAjwg6b0BRBMEiwANd1_SKvZ8lVUeaTdZsvdA....(Accessed:6th April 2020)
2. Arfin, T. & Tarannum, A. Engineered nanomaterials for industrial application: An overview. in Handbook of Nanomaterials for Industrial Applications 127–134 (Elsevier, 2018). doi:10.1016/B978-0-12-813351-4.00006-7
3. Larue, C. et al. Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure. J. Hazard. Mater. 273, 17–26 (2014).
4. Varadan, V. K. Nanomedicine : design and applications of magnetic nanomaterials, nanosensors, and nanosystems. TA - TT - (Wiley, 2008).
5. Latorre, M. & Rinaldi, ; Carlos. Applications of Magnetic Nanoparticles in Medicine: Magnetic Fluid Hyperthermia. prhsj.rcm.upr.edu (2009).
6. Edebali, S., Oztekin, Y. & Arslan, G. Metallic engineered nanomaterial for industrial use. in Handbook of Nanomaterials for Industrial Applications 67–73 (Elsevier, 2018). doi:10.1016/B978-0-12-813351-4.00004-3
7. Nee Koo, K., Fauzi Ismail, A., Hafiz Dzarfan Othman, M., Rahman, M. A. & Zhong Sheng, T. Preparation and characterization of superparamagnetic magnetite (Fe3O4) nanoparticles: A short review. / Malaysian Journal of Fundamental and Applied Sciences 15, (2019).
8. Paliwal, R., Jayachandra Babu, R. & Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. doi:10.1208/s12249-014-0177-9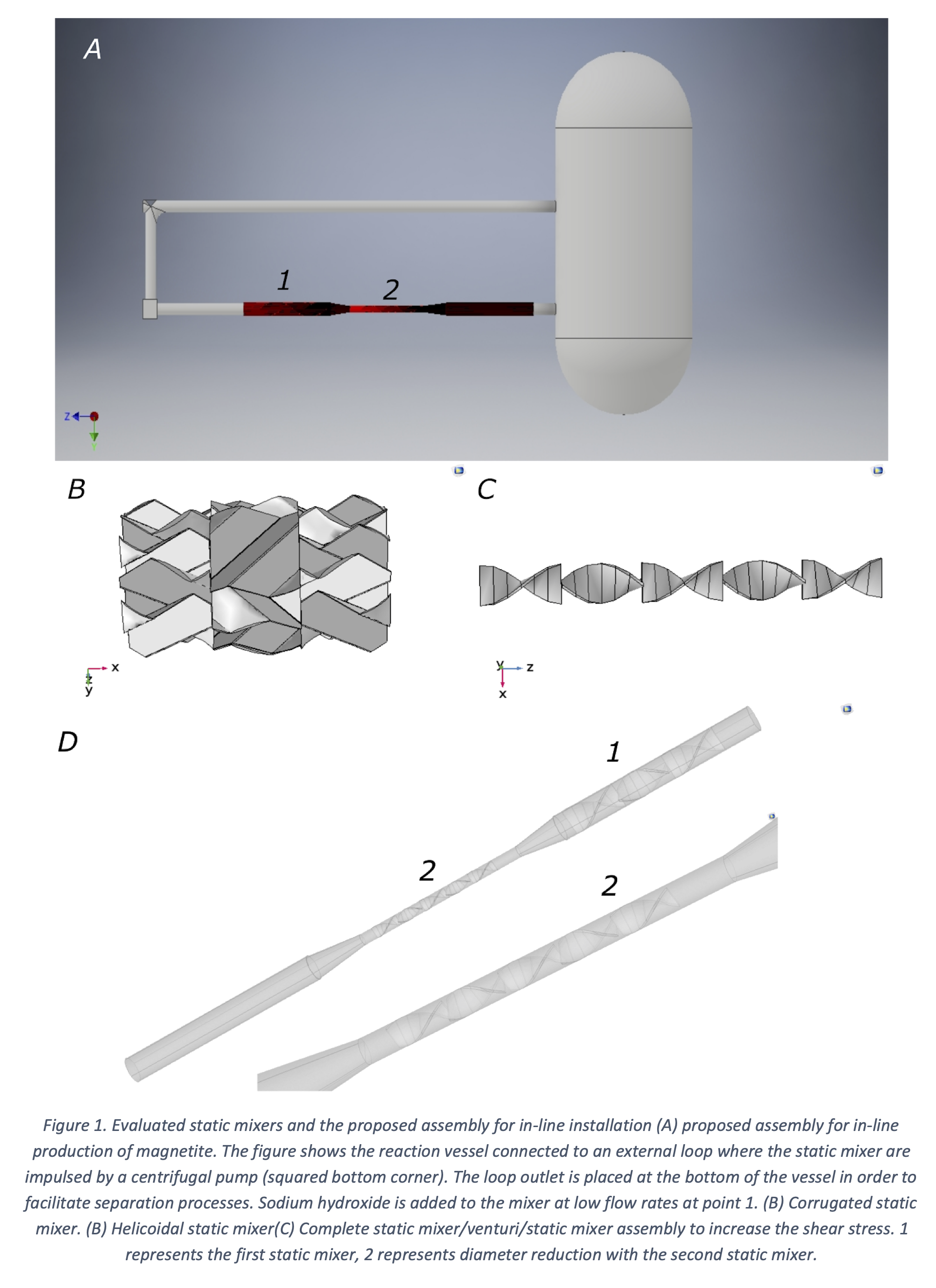
Topics
Checkout
This paper has an Extended Abstract file available; you must purchase the conference proceedings to access it.
Do you already own this?
Log In for instructions on accessing this content.
Pricing
Individuals
| AIChE Pro Members | $150.00 |
| AIChE Emeritus Members | $105.00 |
| AIChE Graduate Student Members | Free |
| AIChE Undergraduate Student Members | Free |
| AIChE Explorer Members | $225.00 |
| Non-Members | $225.00 |
