(548b) NAMF Student Award Presentation 1: Hydrodynamic Characterization of the USP Dissolution Apparatus 1 Under Different Basket Mesh Sizes
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
North American Mixing Forum
North American Mixing Forum Award Session Part 1: Student Award Finalists (Invited Talks)
Friday, November 20, 2020 - 9:00am to 9:20am
Baskets with different mesh sizes can be chosen for such a system in order to discriminate between formulations with different drug release profiles in new drug formulation development. The hydrodynamics generated by the basket controls the dissolution rate and hence the results of the test. Therefore, any changes introduced by different basket geometries or other geometric factors can significantly impact the system hydrodynamics and cause variability of results, thus impacting product quality. Several reports in the literature have indicated that there is appreciable variability and unpredictability in the dissolution profiles obtained in this and other compendial dissolution testing apparatuses. The high sensitivity of the USP Apparatus 1 hydrodynamics to even small variations in the geometry and operating variables appears to be one of the reasons for the variability and inconsistency of test results.
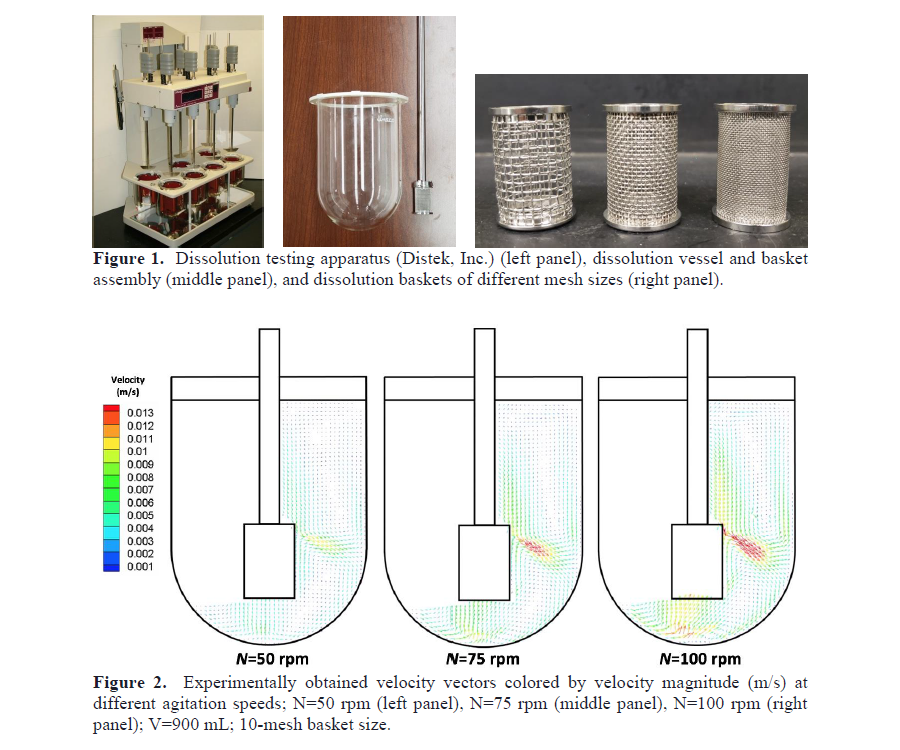
Therefore, the main objective of this work was to quantify the hydrodynamics in a standard USP dissolution vessel Apparatus 1 using an experimental method, i.e., Particle Image Velocimetry (PIV) and a computational approach, i.e., Computational Fluid Dynamics (CFD) approaches, for different basket mesh sizes, different agitation speeds, and different vessel fill levels. The results obtained here (an example of which is shown in Figure 2) indicate that variations in geometric parameters and operating variables within standard criteria appear to significantly affect the hydrodynamics of these systems, thus contributing to the variability of dissolution testing results.
The results of this study can help establish the basis for a more fundamental understanding and, possibly, prediction of the actual dissolution process when combined with additional information about the formulation and the disintegration/dissolution mechanism. Furthermore, a more fundamental understanding of the hydrodynamics of USP Apparatus 1 could possibly result in design improvements and/or modifications of the current compendial monograph, which could ultimately benefit USP, FDA, and industry.
Captions
Figure 1. Dissolution testing apparatus (Distek, Inc.) (left panel), dissolution vessel and basket assembly (middle panel), and dissolution baskets of different mesh sizes (right panel).
Figure 2. Experimentally obtained velocity vectors colored by velocity magnitude (m/s) at different agitation speeds; N = 50 rpm (left panel), N = 75 rpm (middle panel), N = 100 rpm (right panel); V=900 mL; 10-mesh basket size.