(533g) Tumor Acidic Microenvironment Impairs Cancer Cell Migration
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Topical Conference: Chemical Engineers in Medicine
Engineering Cancer
Tuesday, November 17, 2020 - 9:30am to 9:45am
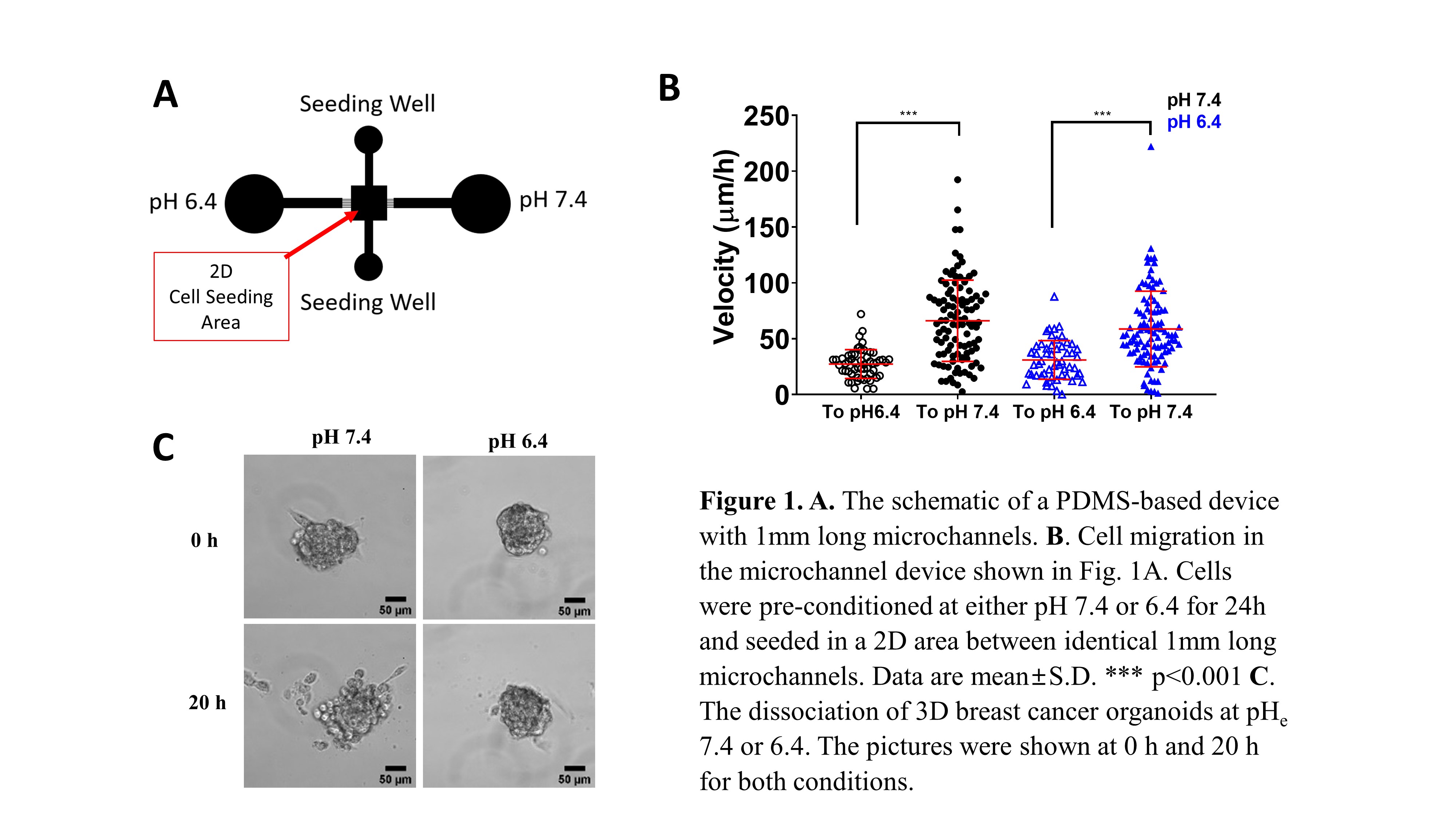
In this study, we employed PDMS-based microfluidic devices to track cell migration inside channels of different dimensions. We also studied 2D migration as well as tumor cell dissociation from 3D breast cancer organoids. In separate experiments, cells were seeded on a 2D area between two sets of 1 mm long microchannels leading to either acidic or normal local microenvironment (Fig. 1A). The effects of acidosis were also investigated using mouse models.
We measured reduced cell motility in 2D and inside microchannels under acidosis. The most pronounced differences were observed at an extracellular pH of 6.4. This pH also interfered with tumor cell dissociation from 3D breast cancer organoids (Fig. 1C). We determined that the reduced migratory capacity of cells grown in an acidic microenvironment is due to decreased mRNA and protein expression of Na+/H+ exchanger, NHE1. Along these lines, we determined that NHE1 activity is also significantly lower under acidosis, as measured by the NH4Cl pre-pulse technique. This is further substantiated by data showing that NHE1 knockdown in cells grown at the pH of 7.4 suppresses motility down to the levels with cells cultured at pH 6.4 for 24 hours. In line with our in vitro findings, breast cancer cells, pre-exposed to acidic pH prior to their injection in the tail vein of mice, exhibited reduced levels of lung colonization. Interestingly, cells seeded on 2D preferentially moved through microchannels toward physiological pH of 7.4 as opposed to pH 6.4 in significantly larger numbers and with faster speeds (Fig. 1B). The tendency of cells to move away from an acidic microenvironment was observed for breast cancer cells that were preconditioned for 24 hours at either pHe 7.4 or 6.4. This result may explain tumor cells’ propensity to leave the primary tumor during metastasis.
We propose that extracellular acidosis impairs cancer cell migration on 2D and in 3D-like microfluidic channels as well as cell dissociation from breast cancer organoids, presumably by downregulating both the expressions and activity of NHE1. Although acidosis impairs cell migration, we posit that cancer metastasis is in part driven by tumor cells’ propensity to migrate away from the acidic tumor environment they create towards physiological pH environments. Our study expands our understanding of cancer cell behavior in acidic tumor microenvironments, which could aid in future cancer research and development of cancer therapeutics.
References
1. S.K. Parks et. al. Nat Rev Cancer, (2013) 13, 611-623.
2. C.D. Paul et. al. Nat. Rev. Cancer (2017) 17,131–140 .
3. S.C. Gupta et. al. Oncotarget, (2014) 5:12070-12082.
4. Y. Wu et. al. Tumor Biol, (2017) Jun; 39(6):1010428317705750.
5. C. Chen et. al., Exp Cell Res, (2010) Oct 15;316(17):2910-21
6. R.D. Castellone et. al., Cancer Letter, (2011) doi.org/10.1016/j.canlet.2011.08.013