(446d) Internal Liquid Crystal Structures in Nanocarriers Containing Drug Hydrophobic Ion Pairs Dictate Drug Release
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Food, Pharmaceutical & Bioengineering Division
Tuning Drug Kinetics
Wednesday, November 18, 2020 - 8:45am to 9:00am
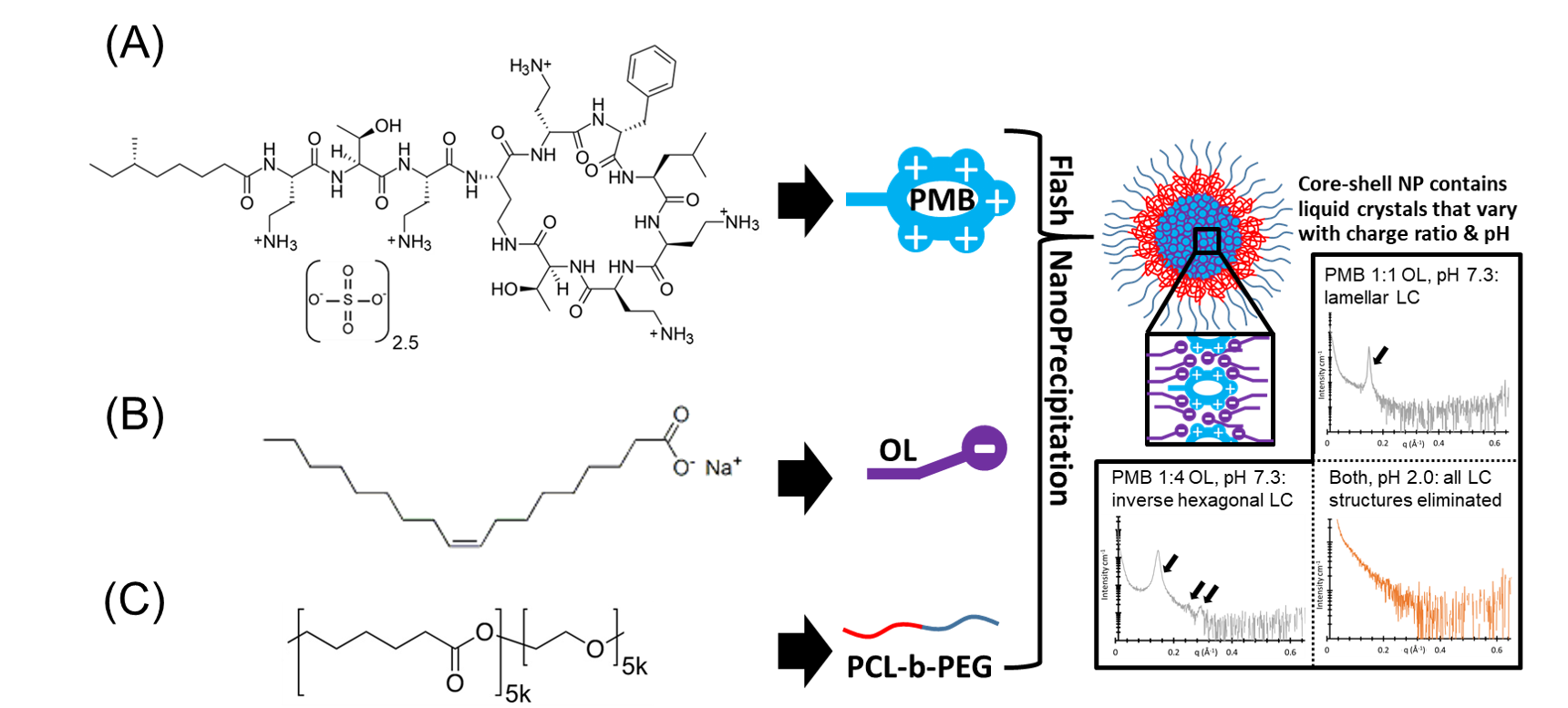
Hydrophobic ion pairing is an attractive strategy for encapsulating and controlling release of charged water-soluble species. Hydrophobic complexes formed this way are sensitive to salts and pH, but the underlying mechanisms and dynamics remain unknown. We here demonstrate that a model hydrophobic ion pair formed from the cationic peptide polymyxin B sulfate and the anionic surfactant sodium oleate forms liquid crystal structures inside the cores of nanoparticles formed using Flash NanoPrecipitation. Internal structures were observed by SAXS and TEM following nanoparticle formulation and were found to vary with polymyxin:oleate charge ratio. Upon exposure to phosphate-buffered saline at pH 7.3, release of encapsulated polymyxin from the nanoparticles was induced. The internal structure was either retained or altered during release, depending on the polymyxin:oleate charge ratio. For a formulation containing a four-fold charge excess of oleate relative to polymyxin, the internal structures rearranged into an inverse hexagonal phase. The hexagonal phase formation corresponded with a greatly reduced rate of polymyxin release, suggesting that the polymyxin was incorporated into the center of hexagonally-packed rods. When release tests were repeated using a PBS at pH 2.0 to ensure protonation of the oleic acid and prevention of ion pairing, all internal structures were eliminated and release occurred much faster than at neutral pH, regardless of charge ratio. These findings shed light on the mechanism behind stimulus-responsive drug release from systems containing hydrophobic ion pairs and could lead to rational design of controlled-release formulations by manipulating the formation and dynamics of liquid crystalline phases inside nanocarriers.