(437a) Determining the Effect of Water during the Catalytic Reduction of Propanal to Alcohols on Ru-Based Catalysts
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Reaction Engineering for Biomass Conversion
Monday, November 16, 2020 - 8:00am to 8:15am
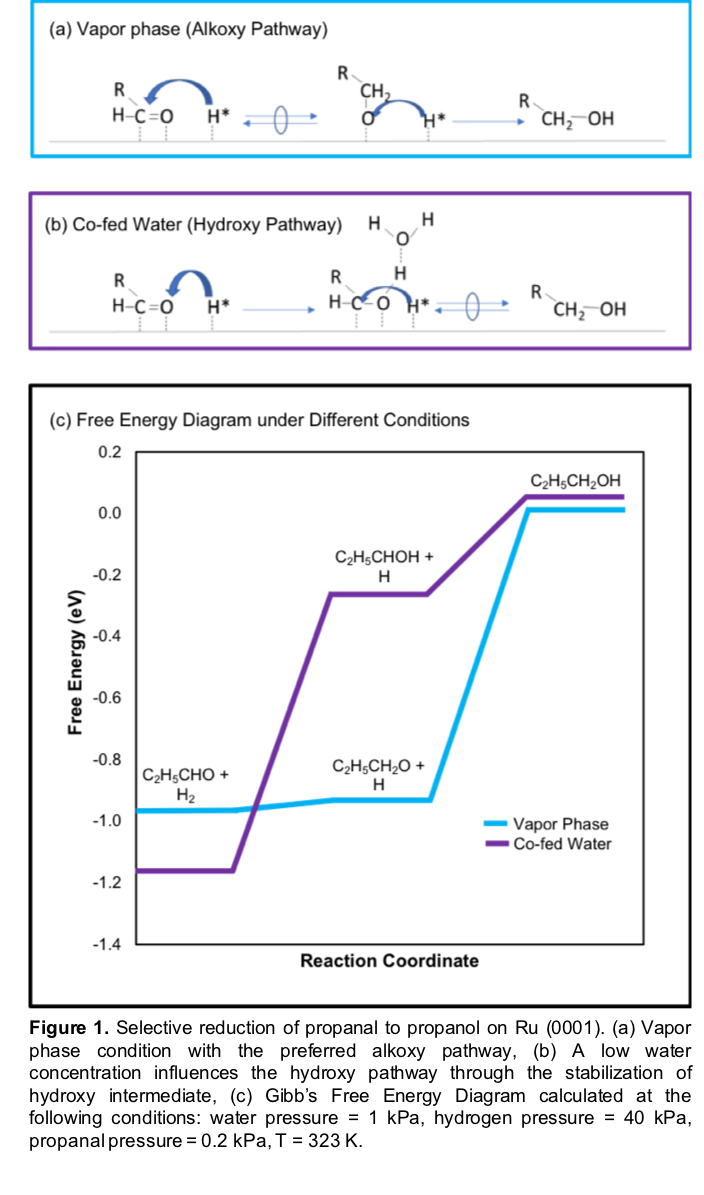
Our results suggest that, in the vapor phase, hydride adspecies are present on the Ru surface and react with adsorbed propanal by preferentially attacking the positively charged carbon to rapidly and selectively form a C-H bond, which results in an Alkoxy pathway (Figure1). Co-feeding water during the reduction of propanal in the vapor phase produces an environment with a low water concentration, which bridges the vapor phase and the condensed water phase. Increasing the water pressures and the number of water molecules around the active sites in our model, induces a switch in the hydrogen addition order by stabilizing the transition state via hydrogen bonding and results in a reduction of the O-H formation barrier and a different hydrogen addition order (Figure 1). Overall, this work provides theoretical and experimental insights demonstrating the systematic tunability of the solvent with regard to the underlying energetics of the elementary steps during the catalytic sequence and the reaction pathway for propanal reduction to alcohols.