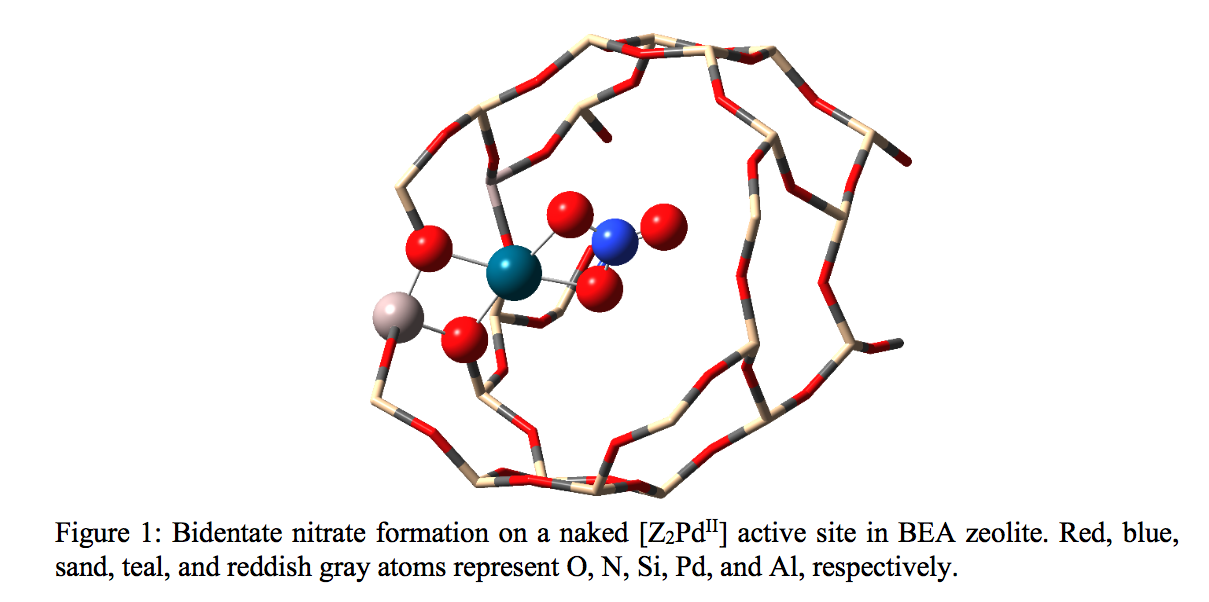
(433h) A DFT Study for the Mechanistic Understanding of Passive NOx Adsorption on Pd/H-BEA Under Dry Condition
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Computational Molecular Science and Engineering Forum
Practical Applications of Computational Chemistry and Molecular Simulation I
Tuesday, November 17, 2020 - 9:45am to 10:00am
In the present work, we have used density functional theory to gain a mechanistic understanding of NOx adsorption on Pd/H-BEA, and have proposed a sequence of feasible elementary steps that can explain observations from flow reactor experiments for NOx trapping.1 Due to the complex nature of palladium speciation in zeolite pores, the structure of the active site remains elusive. For zeolites with atomically dispersed Pd in the absence of water the most likely active sites are [Z2PdII], [ZPdIIOH], and [ZPdI]. On the [Z2PdII] site NO can be stored by reversible molecular adsorption or in the form of nitrate (Figure 1). The [ZPdIIOH] site catalyzes low energy pathways for NO and also CO oxidation, while [ZPdI] can be dynamically formed and provides the strongest NO binding site. The presence of CO as stronger reductant was found to be beneficial for NOx storage. The active site assignments are corroborated by vibrational analysis and comparison to observed experimental diffuse reflectance spectra.1
Capturing the temperature dependent evolution and role of active sites in Pd/H-BEA upon NO exposure will expedite the development of suitable PNA materials to achieve desired emission control performance at low temperature.
Reference:
- Malamis, Sotirios A., Michael P. Harold, William S. Epling, Industrial & Engineering Chemistry Research 58,22912-22923 (2019).