(423d) High Performance Anion Exchange Membranes Based on Block Co-Polymers for Energy Conversion Applications
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Separations Division
Membranes for Electrochemical Conversions and Applications I
Tuesday, November 17, 2020 - 8:45am to 9:00am
One of the main hurdles is balancing the ion exchange capacity (IEC) against water uptake and swelling of the material. A higher IEC will contribute to having a higher ionic conductivity at the cost of increasing the hydration and swelling of the material, which can ultimately lead to mechanical failure. Many of the leading AEMs with high hydroxide ion conductivity (96 – 140 mS cm-1 at 80 °C in liquid water) suffer from excessive water uptake, > 100%, and this can be especially detrimental in liquid applications. Ongoing research has focused on identifying alkali stable polymer backbones and cation groups, since hydroxide ions can attack ionic groups along with the polymer backbone itself through multiple degradations pathways. Numerous cation chemistries have been explored, namely quaternary ammonium groups, of which trimethylammonium has been the most widely investigated. However, trimethylammonium functionalized polymers have been shown to have poor stability at high pH, and have low or no ionic conductivity at elevated temperatures. Marino and Kreuer studied 26 different quaternary ammonium groups at high temperature and pH and found that quaternized aliphatic-heterocycles with 5 and 6 membered rings were much more stable than the traditional trimethylammonium ions, since they were less susceptible to both nucleophilic attack and substitution.
Equally important is the chemistry of the polymer backbone, where it is desirable to have a hydrophobic backbone to diminish water uptake and membrane swelling and minimize, or eliminate, the presence of polar groups that can act as sites of attack by nucleophilic hydroxide anions. For these reasons, polyethylene is a great candidate for an AEM polymer backbone as it is stable under alkaline conditions, is hydrophobic, does not swell in aqueous solutions, is semi-crystalline, and has excellent mechanical properties. Block copolymers have been extensively investigated for use as backbones for AEMs because their structure induces a phase separated morphology, and well-interconnected hydrophilic domains have been shown to enhance water and ion transport through the membrane.
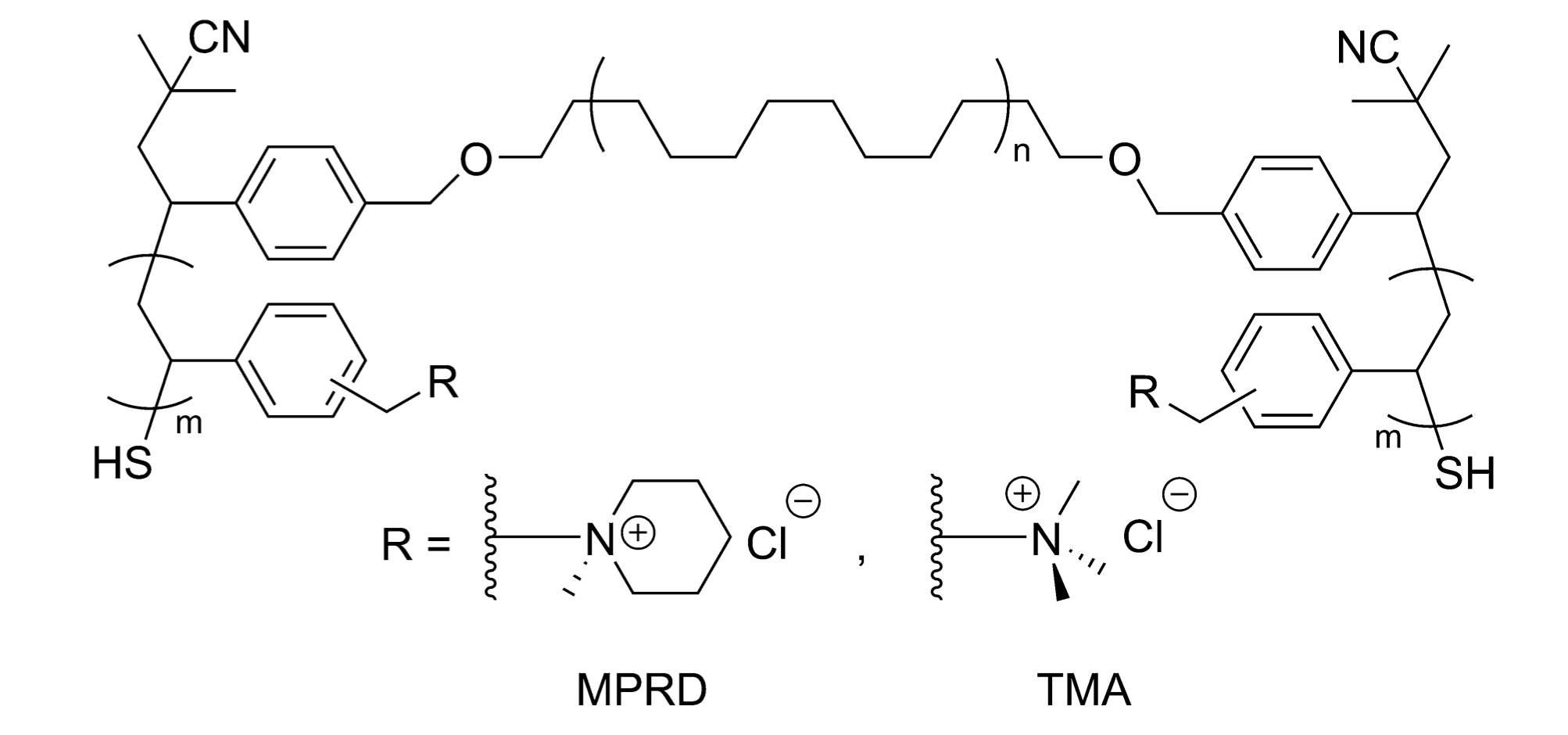
We have developed an ABA triblock copolymer of polychloromethylstyrene-b-polycyclooctene-b-polychloromethylstyrene (PCMS-b-PCOE-b-PCMS) with a scalable and efficient synthesis that was optimized for high ionic conductivity. Based on this previous work, conductivity was shown to increase with increasing lamellar d-spacing. Here we take a PCMS-b-PCOE-b-PCMS triblock precursor and modify it by hydrogenating the polycyclooctene (PCOE) midblock to polyethylene (PE). The ratio of PCMS:PCOE was kept similar to the previous work to ensure good mechanical integrity. Based on the work of Mahanthappa et al., a polydisperse midblock and narrow dispersity outer blocks were synthesized to provide a larger window for nanoscale phase separation. The polyethylene-based triblock copolymer was processed by hot pressing the polymer in a slurry of p-xylene into a film. Hot pressing was performed at two different temperatures, and the elevated temperature lightly crosslinked the PCMS blocks through scission of benzyl chlorides. The films were subsequently quaternized with either trimethylamine to yield benzyltrimethylammonium (TMA) or methylpiperidine to yield benzylmethylpiperidinium (MPRD) cations. The incorporation of PE in the midblock produced anion exchange membranes with enhanced hydroxide and chloride conductivity, in addition to excellent water uptake and interesting mechanical properties.
Based on similar chemistry we have also synthesized ABA polymers with isoprene mid blocks that may be hydrogenated to give amorphous poly(methylbutylene) PMB. In fact we can extend this chemistry for both PE and PMB to give ABABA or BABAB pentablock materials. This additional level of control enables us to totally tune the mechanical properties of these materials for a variety of demanding applications.