(34a) Patient-Specific Supply Chains: Off the Shelf or Made from Scratch?
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Computing and Systems Technology Division
Supply Chain and Logistics
Monday, November 16, 2020 - 8:00am to 8:15am
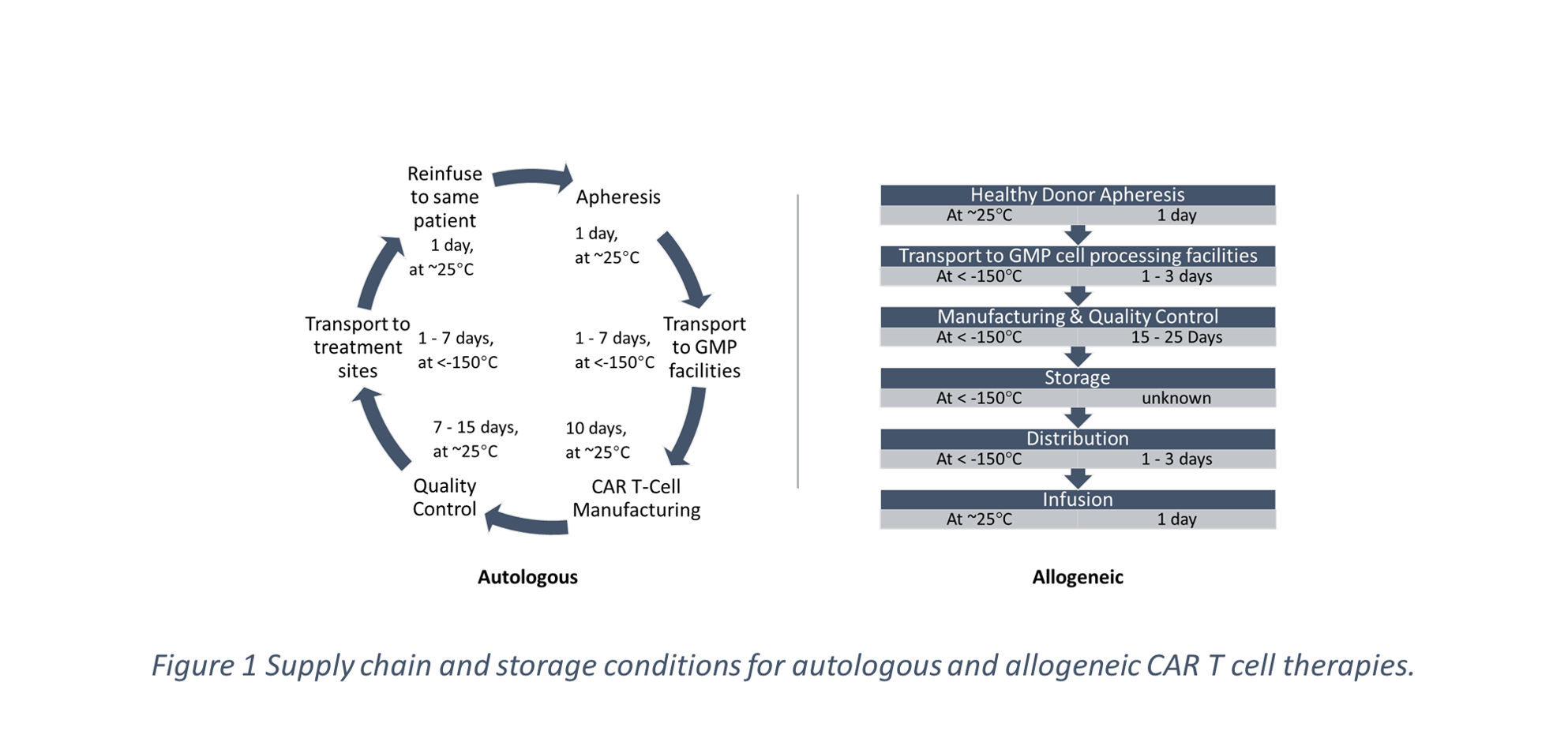
Allogeneic CAR T cell therapies could be a robust alternative to their autologous counterpart as the main raw material (cells) can be off-the-shelf, as it is provided in advance by a compatible donor. This can alleviate risks associated to highly distributed upstream of the supply chain and lead to decreased transport/storage risks. Although no allogeneic CAR T formulation is available yet, a potential approval of such therapies could create a step change in personalised treatments. In this work, we develop Mixed Integer Linear Programming (MILP) models to compare allogeneic and autologous CAR T cell therapy supply chain networks. We investigate different network configurations with increased level of distribution, and we assess their performance with respect to: (a) cost, (b) scalability and (c) total return time of the therapy.
Acknowledgments
Funding from the UK Engineering & Physical Sciences Research Council (EPSRC) for the Future Targeted Healthcare Manufacturing Hub hosted at University College London with UK university partners is gratefully acknowledged (Grant Reference: EP/P006485/1). Financial and in-kind support from the consortium of industrial users and sector organisations is also acknowledged.
References
[1] Novartis, “KYMRIAH Treatment Process, Dosing & Administration | HCP,†2018. [Online]. Available: https://www.hcp.novartis.com/products/kymriah/acute-lymphoblastic-leukem.... [Accessed: 07-Mar-2019].
[2] Kite Pharma, “First CAR T Therapy for Certain Types of Relapsed or Refractory B-Cell Lymphoma,†2018. [Online]. Available: https://www.yescartahcp.com/. [Accessed: 07-Mar-2019].
[3] B. L. Levine, J. Miskin, K. Wonnacott, and C. Keir, “Global Manufacturing of CAR T Cell Therapy,†Mol. Ther. - Methods Clin. Dev., vol. 4, no. March, pp. 92–101, 2017.