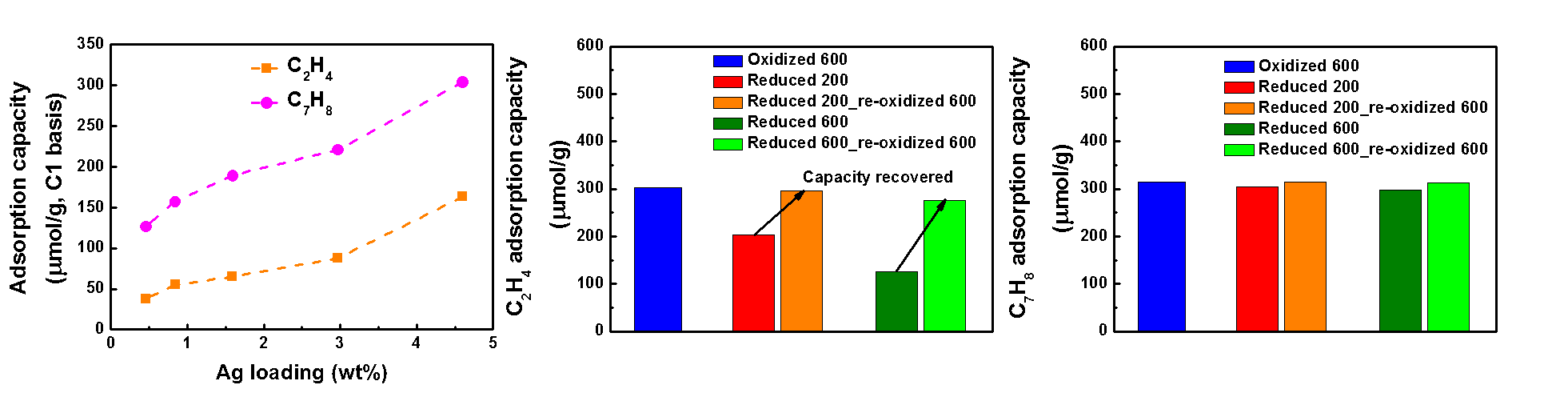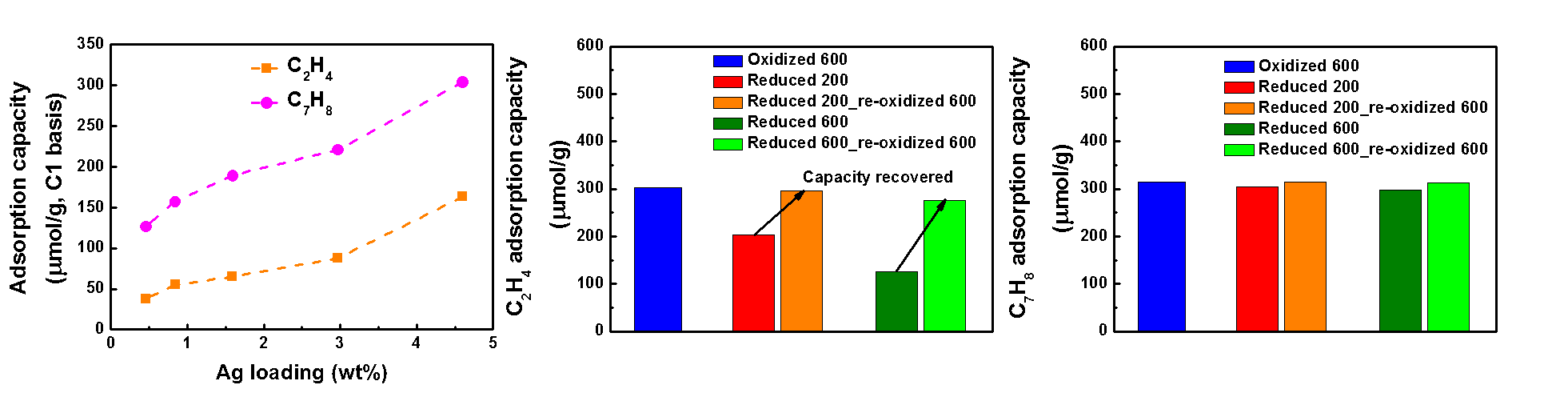(344v) Ag Ion-Exchanged ZSM-5 Zeolites for Hydrocarbons Trapping Applications
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Poster Session: Advances in Zeolite Science and Technology
Friday, November 20, 2020 - 8:00am to 9:00am
Significant attention has been paid to the reduction of vehicle emissions during cold-start due to the stringent emission regulations. A potential approach to regulate the cold-start hydrocarbon (HC) emissions is to utilize HC traps. Light (e.g. C2H4, C3H6) and heavy (e.g. C7H8) HCs need to be trapped simultaneously in practical applications. The goal of this study is to develop suitable HC traps for both light and heavy HCs. Herein, a series of Ag containing ZSM-5 (Si/Al = 11.5) zeolites with different Ag loadings (0.5 - 4.6 wt.%) were prepared via ion-exchange. Their trapping performance was evaluated using the U.S. DRIVE low temperature combustion of diesel (LTC-D) protocol. The results showed that Ag/ZSM-5 is able to adsorb C2H4 and C7H8 simultaneously and their adsorption capacities increased with increasing Ag loading (Fig. 1a). This indicates that Ag is able to act as an adsorption site for both C2H4 and C7H8 in the presence of H2O via π-backbonding. The oxidation state of Ag can also influence HC adsorption (Figs.1b and 1c). Specifically, the initial C2H4 adsorption capacity (303 μmol/g) decreased to 202 and 125 μmol/g after reduction at 200oC and 600oC, respectively. Reduction at 200oC and 600oC resulted to a decrease in the number of Ag+ sites due to Agn+ and Ag0 formation, respectively, and thus a decrease in C2H4 adsorption capacity. The decrease in C2H4 adsorption capacity can be recovered after re-oxidation at 600oC. Unlike C2H4 adsorption, a negligible decrease in C7H8 adsorption capacity was observed after reduction. This behavior can be attributed to the adsorption of C7H8 on both Ag and the zeolite framework, while C2H4 can be adsorbed only on Ag.