(315h) Tuning CO2 Adsorption in Mixed-Metal Metal?Organic Frameworks
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Materials Engineering and Sciences Division
MOF, COF, and Porous Polymer Materials II: Applications
Tuesday, November 17, 2020 - 9:30am to 9:45am
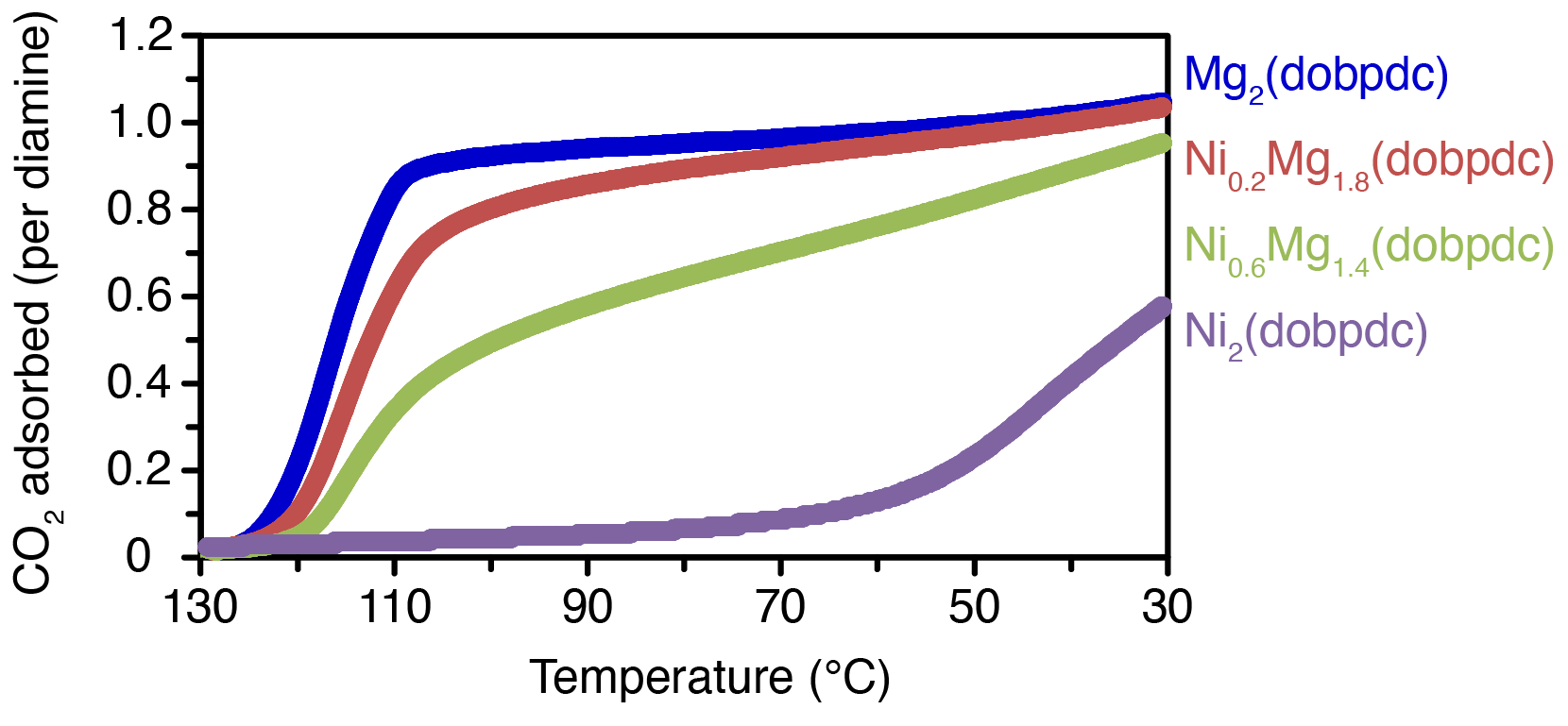
Solid-state magic angle spinning NMR results on the non-diamine appended, 13CO2-dosed M2(dobpdc) MOF suggests that the distribution of metals is roughly homogeneous on the scale of hundreds of nanometers. Additionally, ICP-OES analysis of the synthesized NixMg(2-x)(dobpdc) MOF suggests that there is little to no preferential incorporation of one metal over the other. NMR results for the diamine-appended, 13CO2-dosed e-2-NixMg(2-x)(dobpdc) MOF indicates that the adsorption mechanism is slightly altered in the mixed-metal framework. Finally, thermogravimetric analysis revealed that fine tuning of the adsorption temperatures for varying ratios of metal in the NixMg(2-x)(dobpdc) MOF is feasible and might be applicable for a variety of other metals. Our work thus opens the door for the preparation of designer mixed-metal MOF adsorbents for a wide range of CO2 separations.
Acknowledgement – This work was supported by the College of Chemistry at the University of California, Berkeley under the supervision of Alexander C. Forse and Jeffrey A. Reimer.