(301h) Zinc Anode Design for High-Energy Rechargeable Aqueous Zn-Air Batteries
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Engineering Sciences and Fundamentals
Electrochemical Fundamentals: Faculty Candidate Session I
Monday, November 16, 2020 - 9:30am to 9:45am
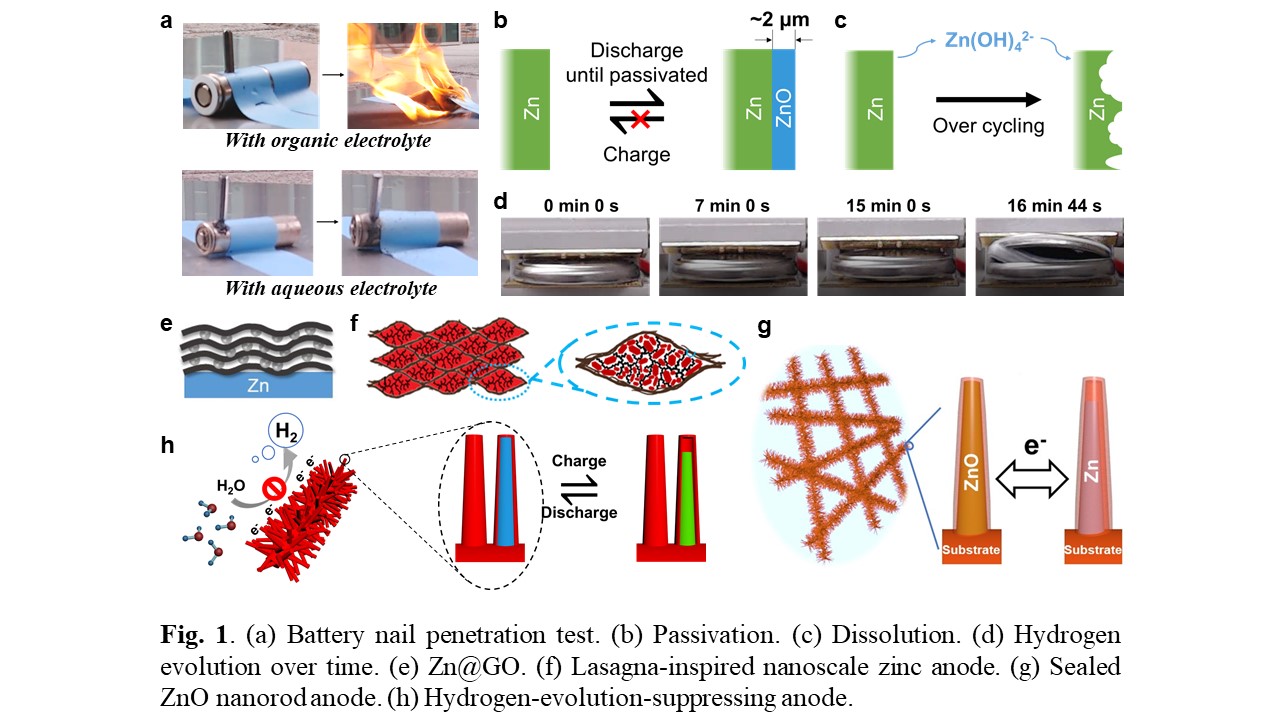
Although stable cycling of Zn anodes in mild acidic electrolyte has been demonstrated, an alkaline electrolyte is ideal for zinc-air batteries, because oxygen cathode has minimum overpotential in alkaline electrolyte. However, the performance of Zn anodes in alkaline electrolyte is limited by passivation (Fig. 1b), dissolution (Fig. 1c) and hydrogen evolution (Fig. 1d) problems. Through SEM investigation, critical passivation size was found to be ~ 2 µm. Thus, sub-micron-sized Zn anodes, with feature size smaller than the critical passivation size, won’t have passivation problem. As a result, we focus our research on nanoscale. However, Zn dissolution and hydrogen evolution of nanosized anodes will be accelerated because of large electrode-electrolyte surface area. I have designed and fabricated 4 types of zinc anodes to control the interface between the electrode and electrolyte by creating interfaces with ion-sieving and hydrogen evolution suppressive properties. These designs (listed below) have successfully overcome passivation, dissolution, hydrogen evolution problems.
- Graphene oxide-modified zinc anode (Zn@GO, Fig. 1e)
- A lasagna-inspired nanoscale zinc anode (ZnO@GO, Fig. 1f)
- Sealed ZnO nanorod anode (Zn@TiNxOy, Fig. 1g)
- A hydrogen-evolution-suppressing anode (Fig. 1h)
All these anodes show superior performance compared with unmodified anodes. These anodes can be paired with air cathodes to make high energy Zn-air batteries. The nanoscale design principles here can potentially be applied to overcome intrinsic limitations of other battery materials. Besides, I will also present my recent research progress on operando optical microscopy analysis of Zn anodes. Dendrite growth and the regulation of Zn metal deposition will be visualized.