(19b) Superacid Polymer Catalysts for Hydroxymethylfurfural Production
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Fundamentals and Strategies for Catalytic Biomass Conversion
Monday, November 16, 2020 - 8:15am to 8:30am
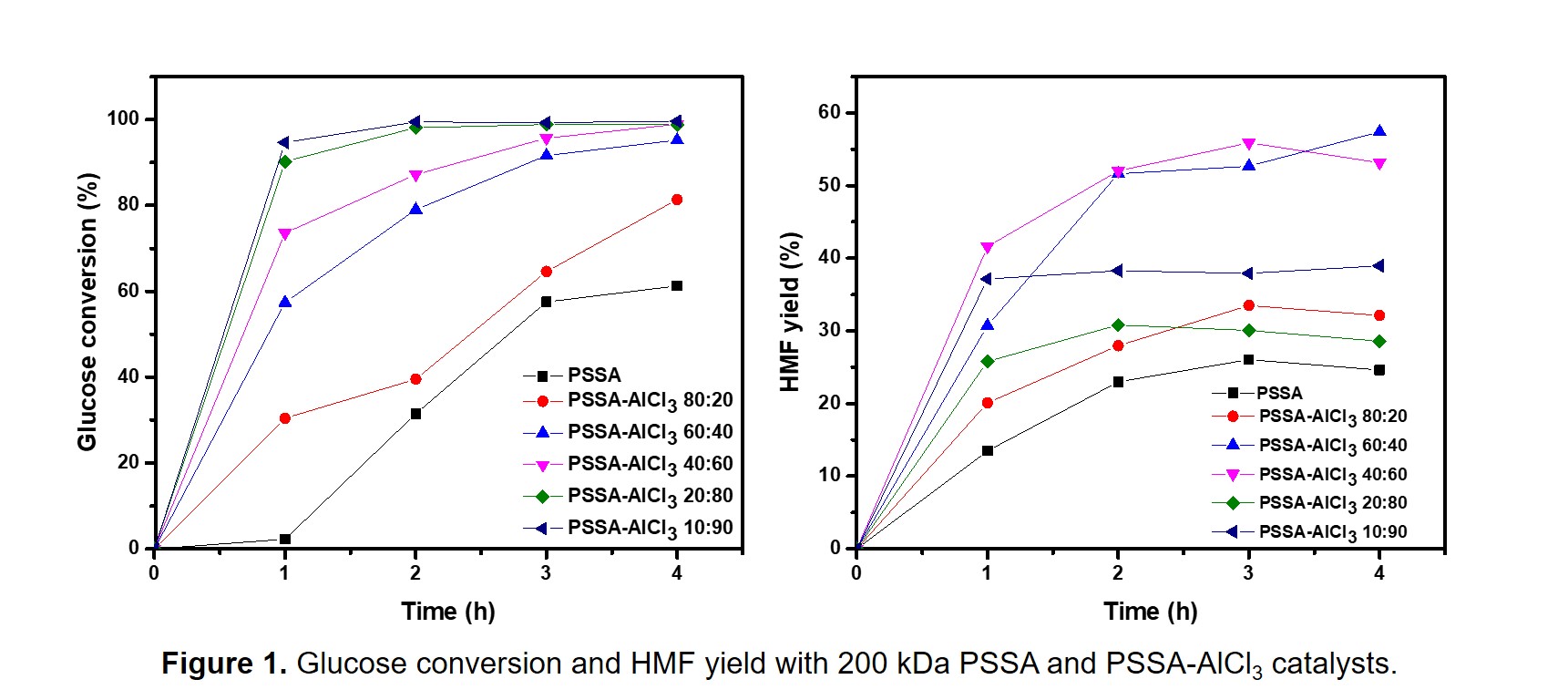
Hydroxymethylfurfural (HMF) is an important and versatile renewable platform chemical. HMF can be obtained from fructose, but its production from glucose is preferred due to its higher availability and lower cost. Glucose is first isomerized to fructose (Lewis acid), followed by subsequent dehydration of fructose to HMF (Brønsted acid). To overcome the challenges associated with the use of homogeneous and heterogeneous catalysts, we have developed novel polymer-based catalysts that join the advantages of both systems. We found that polystyrenesulfonic acid (PSSA), a catalyst containing Brønsted acid sites, was very active in the dehydration of fructose to HMF. PSSA is soluble in polar solvents; therefore, it is very active and cannot be deactivated through coking, with the additional advantage that it can be easily recovered from the reaction medium for further use. First, we explored the effect of the molecular weight on the catalytic activity and recoverability. Then, we added a second functionality (Lewis acidity) to PSSA by ion exchange with AlCl3 to make it suitable for the production of HMF from glucose.1 A series of soluble and reusable catalysts with different Brønsted: Lewis acid ratios was synthesized, characterized, and studied in the tandem catalytic conversion of glucose to HMF.
Figure 1 shows the glucose conversion and HMF yield obtained with PSSA and PSSA-AlCl3 catalysts. The highest HMF yield was obtained with the PSSA-AlCl3 60:40 catalyst, in which 40 % of the Brønsted acid sites were replaced by AlCl3. We found that further addition of AlCl3 resulted in lower HMF yields, likely due to the reduction of the hydrophilicity (and solubility) of the polymer catalysts.
(1) A.C. Alba-Rubio, M.R. Coleman, S. Kalidindi, A.S. Joshi, I.S. Omodolor; Homogeneous and Reusable Superacid Polymer Catalysts Useful for the Synthesis of 5-Hydroxymethylfurfural from Glucose. WO 2019/089448 A1 (2019).