(162j) Formulation and Characterization of Gelatin-Based Hydrogels for Encapsulation of Kluyveromyces Lactis: Applications in Packed-Bed Reactors and Probiotics Delivery
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Materials Engineering and Sciences Division
Poster Session: Materials Engineering & Sciences (08B - Biomaterials)
Thursday, November 19, 2020 - 8:00am to 9:00am
Accordingly, the main objective of the present study was to develop a cross-linked polymeric matrix from commercially available gelatin type A, to encapsulate the lactic acid-producing yeast K. lactis. The thermal, rheological, and mechanical properties, as well as the microscopic features of the prepared matrices, were evaluated before encapsulating cells. Upon encapsulation, a proof-of-concept experiment was performed on a bioreactor and simulated gastrointestinal medium (for saliva, stomach and intestine), where yields, cell viability, and biocompatibility were determined. The bioreactor experiments were conducted on a milliliter-scale, external-loop, airlift system.
The gels were prepared by mixing 3.0, 5.0 and 7.5% (w/v) gelatin with 1.0%, 3.0% and 5.0% (w/w) of the chemical crosslinker glutaraldehyde. The crosslinking reaction was conducted under mechanical agitation between 100 and 200 RPM, and at a temperature of 40 °C for 4 hours. A two-factor experimental design was performed here with 3 levels (32), where the factors were the concentration of glutaraldehyde and the concentration of gelatin in the gel, including samples without the addition of crosslinker. Near (NIR) and far-infrared (IR) spectroscopy were used to identify the functional groups present in the hydrogels. In this case, we tracked NH2, NH, CH2, CH3, H2O, CN, and amide groups in bands in the range of 800 to 2,500 nm (NIR), and 800 to 2,000 nm for the infrared (IR).
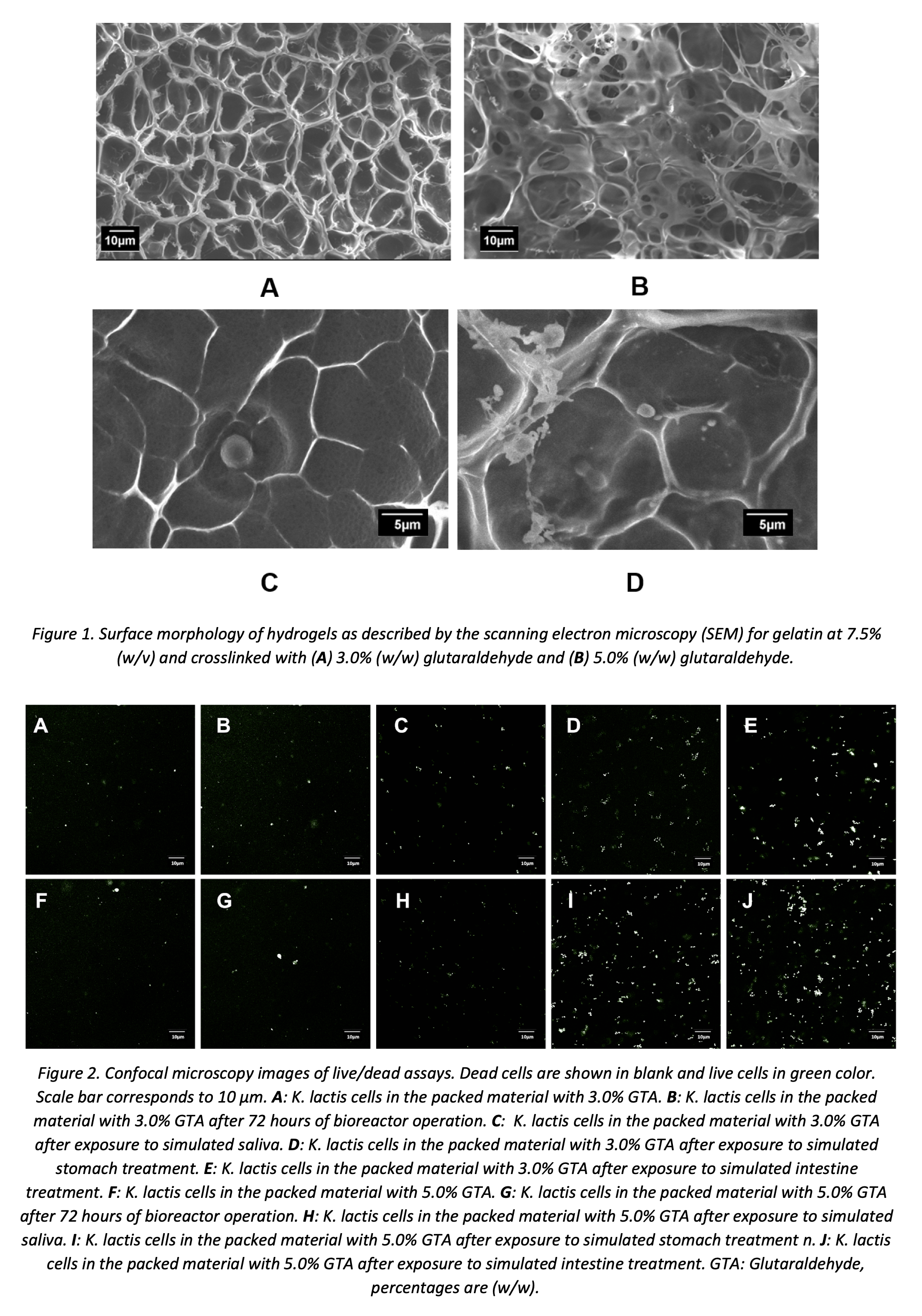
SEM micrographs allowed us to estimate the average pore size, which is a critical parameter to determine the viability of yeast cells encapsulated. The analyses confirmed that an increase in the concentration of glutaraldehyde leads to a decrease in the average pore size of the gel. Gels to continue with cell encapsulation were those with average pore size values in the range between 3 and 8 μm as is presented in Figure 1 (A and B). The same materials were imaged by SEM for direct observation of cells fixed on the hydrogel surface. Moreover, Figure 1 (C and D) shows that the pores are likely to act as microchambers to house the cells that, thereby reserving an essential space for survival and even proliferation during incubation.
Thermogravimetric analyses were conducted in the range from room temperature to 800 ºC to estimate the thermal stability of the gels. After an initial weight loss at about 100 °C (due to water), the gelatin network decomposed after 270 °C. Our results suggest a slight increase in thermal resistance for hydrogels with higher crosslinking levels.
Hydrogels were subjected to swelling in an aqueous medium at pH 7.0. Higher swelling was achieved for gels with lower crosslinking degrees. Additionally, superior structural stability in such medium (about six days) was observed for gels with a 7.5% (w/v) collagen content.
Bloom tests on the gels indicated that an increase in the crosslinking agent concentration led to more stable and elastic materials, however; such an increase in stiffness promoted an increase in the tendency to fracture when subjected to plastic deformation. After milli-bioreactor operation, the original firmness was halved for both cross-linker concentrations. In contrast, after the simulated saliva treatment, the firmness increased most likely due to the incorporation of salts from the medium. Similarly, the simulated stomach medium led to increased firmness most likely due to protonation of superficial groups by the low pH. Finally, the small intestine simulated medium is again pH neutral and therefore promotes the dissociation of the material. The behavior of the material in the whole GI tract is favorable since it manages to overcome the stomach without major structural damage, and becomes penetrable as it reaches the small intestine where the probiotic cells are finally released.
Yeast probiotics cells were grown in a Yeast Nitrogen Base (YNB) supplemented with glucose and lactose culture medium. Encapsulation of K. lactis proceeded in gelatin hydrogels with 7.5% (w/v) collagen contents at 3% and 5% (w/w) glutaraldehyde. Cell viability was evaluated by direct live/dead staining and observation and counting under a confocal microscope. Figure 2 indicated high viability after encapsulation and even after milli-bioreactor operation. When the hydrogels are subjected to the different GI treatments (i.e., saliva, stomach, and small intestine); however, cell viability progressively decreases as it moves along the GI tract. Nevertheless, only 50% of probiotic cells of the encapsulated cells lost their viability.
The approach presented here provides a suitable route for the preparation of chemically-crosslinked gelatin hydrogels with applications in probiotics encapsulation. The obtained encapsulates showed high cell viability in a mili-bioreactor operation and various media corresponding to GI tract compartments.
References
[1] Clare M. Hasler, Functional Foods: Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health, The Journal of Nutrition, Volume 132, Issue 12, December 2002, Pages 3772–3781
[2] C.I. Onwulata. Encapsulation of New Active Ingredients. Annual Review of Food Science and Technology 3, 183–202 Annual Reviews, 2012.
[3] Qiu-Yue Dong, Meng-Yan Chen, Yang Xin, Xue-Yan Qin, Zhuo Cheng, Lu-E Shi, Zhen-Xing Tang. Alginate-based and protein-based materials for probiotics encapsulation: a review. International Journal of Food Science & Technology 48, 1339–1351 Wiley, 2013.
[4] Min Gu, Zipei Zhang, Che Pan, Timothy R. Goulette, Ruojie Zhang, Gregory Hendricks, David Julian McClements, Hang Xiao. Encapsulation of Bifidobacterium pseudocatenulatum G7 in gastroprotective microgels: Improvement of the bacterial viability under simulated gastrointestinal conditions. Food Hydrocolloids 91, 283–289 Elsevier BV, 2019.
[5] Vieira da Silva, Beatriz, et al. “Natural Phytochemicals and Probiotics As Bioactive Ingredients for Functional Foods: Extraction, Biochemistry and Protected-Delivery Technologies.†Trends in Food Science & Technology, vol. 50, 2016, pp. 144–158.
[6] I. B. Holcberg y P. Margalith , «Alcoholic Fermentation by Immobilized Yeast at High Sugar Concentrations,» European J Appl Microbiol Biotechnol , vol. 13, pp. 133-140, 1981.
[7] Yang Li, Chao Feng, Jing Li, Yuzhi Mu, Ya Liu, Ming Kong, Xiaojie Cheng, Xiguang Chen. Construction of multilayer alginate hydrogel beads for oral delivery of probiotics cells. International Journal of Biological Macromolecules 105, 924–930 Elsevier BV, 2017.