(158q) The Relevance of Simulated Lung Fluid Composition on the Drug Solubility and Predicted in-Vivo performance of Inhaled Drug Delivery
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Food, Pharmaceutical & Bioengineering Division
Poster Session: Engineering Fundamentals in Life Science
Tuesday, November 17, 2020 - 8:00am to 8:55am
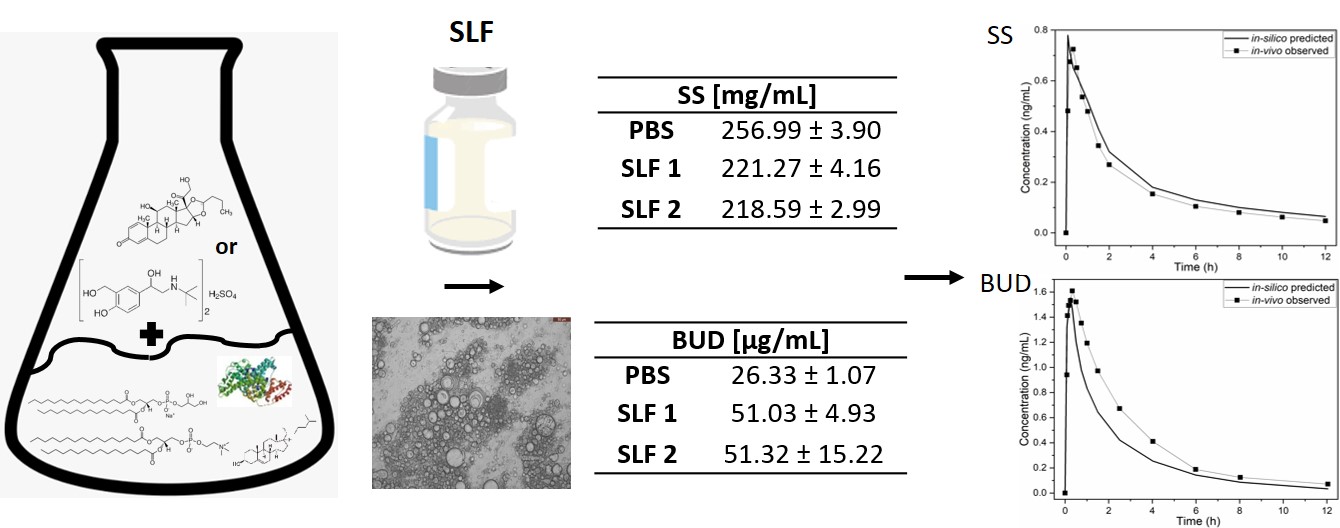
Physiologically based pharmacokinetic modelling (PBPK) is one of the in-silico methods that allows establishment of in-vitro-in-vivo correlations (IVIVC) based on the mechanistic understanding of the physiological processes affecting drug disposition in-vivo. IVIVC aims to enable the correlation between the in-vitro behavior of the drug and its in-vivo performance. PBPK models aim to be able to accurately predict the drug PK in a target organ, based on different parameters such as its physicochemical characteristics, solubility, dissolution, permeability and metabolism but also on the anatomy and physiology of the target patient population[1]. However, in the case of inhaled drug delivery, the clinical relevance of the in-vitro methodologies currently being applied are still not well established. It is necessary, for example, to standardize a simulated lung fluid (SLF) to be used in the solubility and dissolution testing of orally inhaled products and obtain reproducible systematic sets of data able to be confidently applied to drug development. However, presently, this is not yet the case and diverse SLF media are based on the simplistic employment of phosphate buffer (PBS) in the presence of surfactants such as sodiumsodecylsulphate (SDS) or Tween 80[2]. Considering that the lung fluid is composed of surfactant lipids and proteins, PBS based media might not be able to completely capture environment in the lung[3]. Therefore, in this work by using an in-vitro-in-silico feedback-feedforward approach (Figure 1), we aimed to gain a systematic understanding of how different SLF components (i.e. lipids and proteins) can impact drug solubility. Two model inhaled drugs with different lipophilicities were selected to be studied, salbutamol sulphate (SS; logP=0.44) and budesonide (BUD; logP=2.52). Subsequently, the experimental values obtained for the solubility in the distinct SLFs were used as inputs in the PBPK models of the two drugs, resulting in different plasma concentration-time profiles.
Materials and Methods
The solubility of SS and BUD was investigated in PBS buffer (pH=7.40) and in SLF, by adding 5 mL of the media to an excess amount of the drug (n=3). The suspensions were conditioned at 37°C and 100 rpm, for 24h. After that, they were filtered and the drug in solution was quantified using high performance liquid chromatography (HPLC). The media simulating the lung fluid were prepared using lipids – dipalmitoylphosphatidylcholine (DPPC; Avanti Polar Lipids, USA), cholesterol (Sigma Aldrich, USA), dipalmitoylphosphatidylglycerol (DPPG; Avanti Polar Lipids, USA) and proteins – albumin (Sigma Aldrich, USA); alone or in combination.
SLF preparation
The SLF was prepared by weighing the lipids i.e. DPPC (4.60 mg/mL), DPPG (0.50 mg/mL), cholesterol (0.10 mg/mL), and dissolving them in a chloroform methanol (2:1) mixture. The solvents from the solutions were evaporated in a rotavapor (Heidolph Laborota 4000 efficient) at 50°C and 60 rpm to yield lipid bilayers. The lipid bilayers were hydrated using pure PBS buffer or PBS containing albumin. Likewise, different SLFs were obtained – SLFs containing only lipids and proteins and a combination of thereof. Albumin was tested in two concentrations, 13.0 mg/mL and 29.1 mg/mL, as present in the alveolar (AF) and bronchiolar fluid (BF), respectively[3]. This way, the systematic investigation of the impact of each component was possible.
PBPK model development and predictions
Based on the reported in-vivo data for the dry powder inhalers, Diskus®[4] and Turbuhaler®[5], the PBPK models for SS and BUD were respectively developed. The PCAT (Pulmonary Compartmental Absorption and TransitTM) module of GP (GastroPlus; Simulations Plus, USA; version 9.7) was used to develop the PBPKs. To predict the deposition patterns of SS[6] and BUD[7], their aerodynamic size distributions and spirometry profiles were used as inputs in MPPD (Multiple-Path Particle Dosimetry, Applied Research Associates, Inc.).
Results and Discussion
Considering the necessity of having a reliable, reproducible and cost effective SLF, one of our goals was to investigate the impact of lipids and proteins on drug solubility. Solubility values of SS obtained in all the media were similar. The solubility in PBS was very high as well as in SLF containing only lipids. The presence of albumin alone or in combination with lipids (AF) in the media showed no effect on SS solubility. However, in the case of BUD, significant impact of the media components was observed and the solubility values increased in the following order: PBS < SLF with albumin AF ≈ SLF with albumin BF < SLF with lipids ≈ SLF with lipids and albumin AF. The presence of albumin alone increased the solubility almost two-fold, however no difference was observed at the two different used concentrations of the lipid (i.e. AF and BF). The use of lipids increased the solubility the most. Similar results have been reported for BUD when this was tested using the commercial natural surfactant Survanta®[8]. Consequently, it becomes evident that SS and BUD exhibit different solubility behaviors when in the presence of lipids and proteins, that is BUD is impacted and SS not. The different solubility values were used as inputs in the PBPK models of the two drugs and the impact on the PK parameters investigated. The developed pulmonary models showed a total mean absolute percentage error (MAPE) of 25% and 20% as well as a correlation factor of 0.86 and 0.89 for BUD and SS, respectively. Hence, both models were found fit to use for further predictions. As expected, negligible differences in solubilities did not impact the PK parameters of SS. This confirmed that SS disposition is not solubility limited. Nevertheless, in the case of BUD, a more pronounced impact was noted when the solubilities in different media were used. For instance, the predicted cmax and tmax had lower MAPE values when albumin was present in the system, while in case of area under the curve (AUC0-∞) the lowest error was seen when lipids were used. These interesting observations in the PK parameters imply that for BUD a middle value between its solubility in the alveolar and bronchiolar regions must be considered in order to more closely reflect the situation in-vivo.
Conclusion
The present study showed the relevance of carefully selecting the components present in the media used as SLF and highlighted how PBPK modelling can help the development of in-vitro methods and vice versa. We observed that for freely soluble APIs, like SS, simple media, such as PBS, are enough to investigate solubility and proceed to other stages of the biopharmaceutical evaluation. For BUD, the greater influence of the media components on solubility, and the different outcomes in terms of the predicted PK parameters, imply that in the case of poorly soluble APIs a more careful consideration must be applied to the in-vitro testing protocols. To support our observations, further studies should be carried out in order to screen a larger variety of molecules.
References
[1] P. Bäckman, S. Arora, W. Couet, B. Forbes, W. de Kruijf, and A. Paudel, “Advances in experimental and mechanistic computational models to understand pulmonary exposure to inhaled drugs,†Eur. J. Pharm. Sci., vol. 113, no. June 2017, pp. 41–52, 2018.
[2] S. Radivojev, S. Zellnitz, A. Paudel, and E. Fröhlich, “Searching for physiologically relevant in vitro dissolution techniques for orally inhaled drugs,†Int. J. Pharm., 2018.
[3] E. M. Bicer, “Compositional characterisation of human respiratory tract lining fluids for the design of disease specific simulants,†2015.
[4] A. Moore, K. Riddell, S. Joshi, R. Chan, and R. Mehta, “Pharmacokinetics of Salbutamol Delivered from the Unit Dose Dry Powder Inhaler: Comparison with the Metered Dose Inhaler and Diskus Dry Powder Inhaler,†J. Aerosol Med. Pulm. Drug Deliv., vol. 30, no. 3, pp. 164–172, 2017.
[5] S. Lähelmä et al., “Equivalent lung dose and systemic exposure of budesonide/formoterol combination via easyhaler and turbuhaler,†J. Aerosol Med. Pulm. Drug Deliv., vol. 28, no. 6, pp. 462–473, 2015.
[6] J. N. Seheult et al., “The acoustic features of inhalation can be used to quantify aerosol delivery from a DiskusTM dry powder inhaler,†Pharm. Res., vol. 31, no. 10, pp. 2735–2747, 2014.
[7] Ã. Farkas et al., “Numerical simulation of emitted particle characteristics and airway deposition distribution of Symbicort® Turbuhaler® dry powder fixed combination aerosol drug,†Eur. J. Pharm. Sci., vol. 93, pp. 371–379, 2016.
[8] D. Palmer, S. Schürch, and J. Belik, “Effect of budesonide and salbutamol on surfactant properties,†J. Appl. Physiol., vol. 89, no. 3, pp. 884–890, 2000.
Figure 1: Design of the study, with examples of solubility results and developed PBPK models