(157bl) Computational Modeling for Designing a Device Harboring Optogenetically Engineered Pancreatic Beta-Cells
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Food, Pharmaceutical & Bioengineering Division
Poster Session: Bioengineering
Tuesday, November 17, 2020 - 8:00am to 9:00am
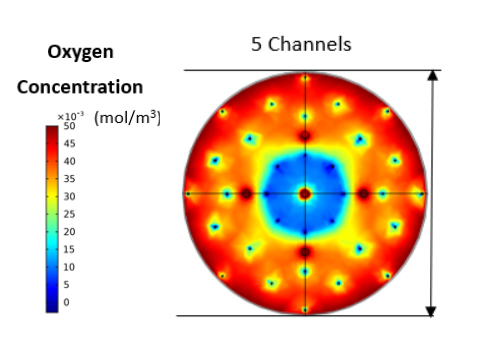
A reaction-diffusion model was built for a disc- or cylinder-shaped construct incorporating the LED source for photostimulated augmentation of GSIS [1][2]. The physical and optical properties of the construct were considered. The production or consumption rate of pertinent species such as glucose and oxygen were modeled with Michaelis-Menten kinetics [3]. To calculate the distribution of oxygen within the scaffold, boundary conditions were applied around the hydrogel and a no-flux condition was enforced in the area adjacent to the LED. Various numbers of cells were placed evenly in the scaffold as 100-150 µm clusters or pseudoislets (PIs). Almost 30% of PIs experienced hypoxia prompting the introduction of channels to alleviate this issues. Indeed, substantial improvement in oxygen distribution and minimal hypoxia within the hydrogel were observed when channels were positioned symmetrically around the vertical axis. The insulin flux in the current design was calculated based on the insulin secretion rate of PAC-expressing beta-cells with or without stimulation with light under different glucose concentrations.
This framework will help gain deeper insights for the cost-effective design of hydrogel constructs with appropriate geometries and cell seeding densities without compromising viability, optogenetic stimulation and function. Our approach is applicable to a range of emerging technologies capitalizing on advances in the field of optogenetics.
Acknowledgements
This work was supported by a grant (CBET-1951104) from the National Science Foundation.
References
- Zhang, E. S. Tzanakakis, Amelioration of Diabetes in a Murine Model upon Transplantation of Pancreatic β-Cells with Optogenetic Control of Cyclic Adenosine Monophosphate, ACS Synth. Biol.8(10): 2248-55, 2019.
- Zhang, E.S. Tzanakakis, Optogenetic regulation of insulin secretion in pancreatic β-cells, Sci. Rep.7(1): 9357, 2017.
- Wu, et al., Oxygen transport and stem cell aggregation in stirred-suspension bioreactor cultures, PLoS One 9(7): e102486, 2014.