(136d) Synthesis of Metal Chalcogenide Nanoparticles and Micro-Assemblies Using Facile Solvent Chemistry
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Materials Engineering and Sciences Division
Graduate Student Award Session (Area 08D)
Monday, November 16, 2020 - 8:45am to 9:00am
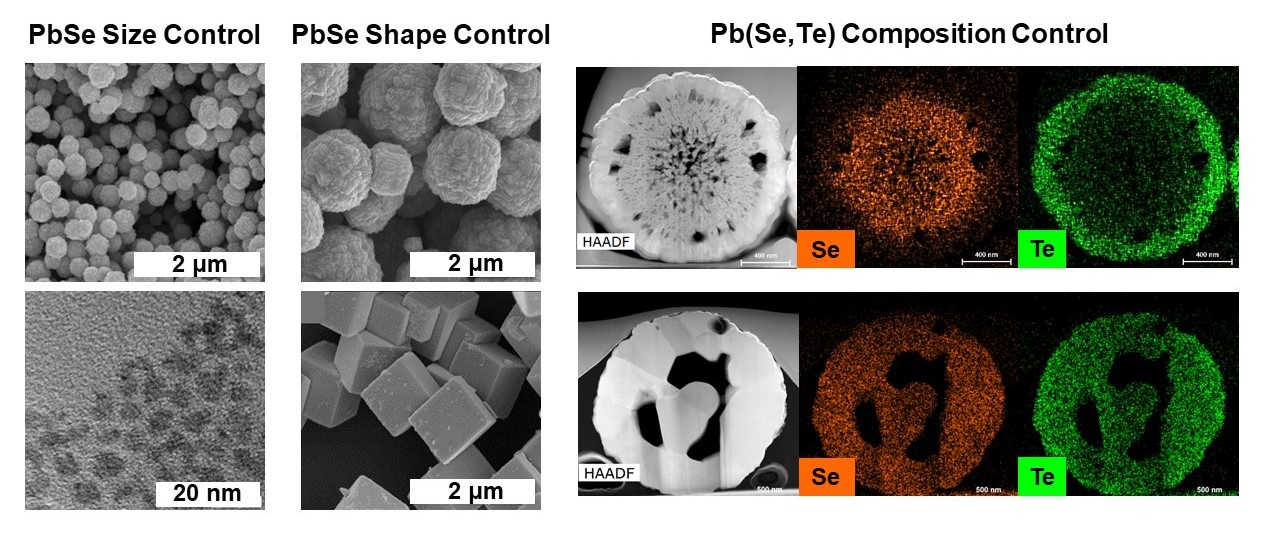
Synthesis of lead chalcogenide particles was realized by dissolution and room-temperature reaction between lead halide solution and chalcogen solution in amine-thiol solvent system.2 With appropriate selection of amine-thiol pair and their relative ratios, the size and shape of the resulting nanoparticles and their self-assemblies were controlled without introducing any additional ligand or temperature variation. Due to the use of volatile amine-thiol solvents in these reactions, the organic residue on the particles was also eliminated making it attractive for use in electronic applications. PbS material synthesized via this facile route demonstrated quantum confinements in nanoparticle as well as assembly structures. This chemistry also allowed for solution-phase cation and anion exchanges in the PbTe system at room temperature which resulted in controlled composition variation within the micro-structures. While PbSe nanoparticles demonstrated promising thermoelectric performance, the structural analysis of PbTe microparticles showed the presence of hollow-core which provides a possible route for a reduction in thermal conductivity and improvement in thermoelectric performance of the material.
While lead chalcogenide particles were synthesized using the room-temperature route, other binary, ternary and quaternary metal chalcogenides such as Cu2S, (InxGa1-x)2S3, CuIn(S,Se)2, CuInxGa1-xS2 and Cu2ZnSnS4 were synthesized at higher temperatures (150-300 oC) using a similar solvent system.3 Precursor solutions used for these reactions were obtained from the dissolution of pure metals like Cu, In, Sn, Zn, Ga, and Se in amine-dithiol solution. The speciation of metal thiolates formed upon the dissolution of metal precursors and its decomposition to phase pure chalcogenide material was studied via X-ray absorption, XRD, Raman, 1H-NMR, and Mass spectrometry.4 Due to the use of elemental metals, this approach completely avoids anionic impurities that metal chloride, iodide, acetate, nitrate, and acetylacetonate salts can introduce in the reaction making it a true impurity-free route for nanoparticle synthesis. These syntheses of nanoparticles were also carried out in setups like heat up, hot injection and microwave-assisted solvothermal conditions giving versatility of experimental settings for better control over particle properties like size, shape, and phase. Amongst these material systems, the CuInxGa1-xS2 nanoparticles synthesized from this chemistry were further used for fabrication of the PV device which yielded a promising power conversion efficiency of around 12%.
As numerous other metal precursors (precursors of Ag, Bi, As, Sb, Ba, etc.) are soluble in amine-thiol solutions, the reaction routes proposed in our work can be extended to these solutions for the synthesis of a variety of other metal chalcogenide nanoparticles.
(1) McCarthy, C. L.; Brutchey, R. L. Chem. Commun. 2017, 53 (36), 4888–4902.
(2) Miskin, C. K.; Deshmukh, S. D.; Vasiraju, V.; Bock, K.; Mittal, G.; Dubois-camacho, A.; Vaddiraju, S.; Agrawal, R. ACS Appl. Nano Mater. 2019, 2, 1242–1252.
(3) Deshmukh, S. D.; Ellis, R. G.; Sutandar, D. S.; Rokke, D. J.; Agrawal, R. Chem. Mater. 2019, 31 (21), 9087–9097.
(4) Zhao, X.; Deshmukh, S. D.; Rokke, D. J.; Zhang, G.; Wu, Z.; Miller, J. T.; Agrawal, R. Chem. Mater. 2019, 31 (15), 5674–5682.