(126f) Effect of Electric Field on Kinetic Modeling of the ORR: Rationalizing pH Behavior of Metal and Oxide ORR Catalysts
AIChE Annual Meeting
2020
2020 Virtual AIChE Annual Meeting
Catalysis and Reaction Engineering Division
Electrocatalysis I: Oxygen
Monday, November 16, 2020 - 9:15am to 9:30am
Next, we show that this simple kinetic model can also explain experimental trends seen on oxide catalysts used in the ORR. By considering subtle differences in the O vs OH scaling relation as well as the effect of electric field, we are able to explain why researchers have struggled to find oxide catalysts which compete with platinum for 4e-ORR activity in acid, as well as why oxide catalysts tend to be more selective toward the 2e-ORR than similar metal catalysts. Ultimately, we show that that this updated kinetic model can help explain experimentally observed catalytic trends, as well as help predict future oxygen reduction catalysts.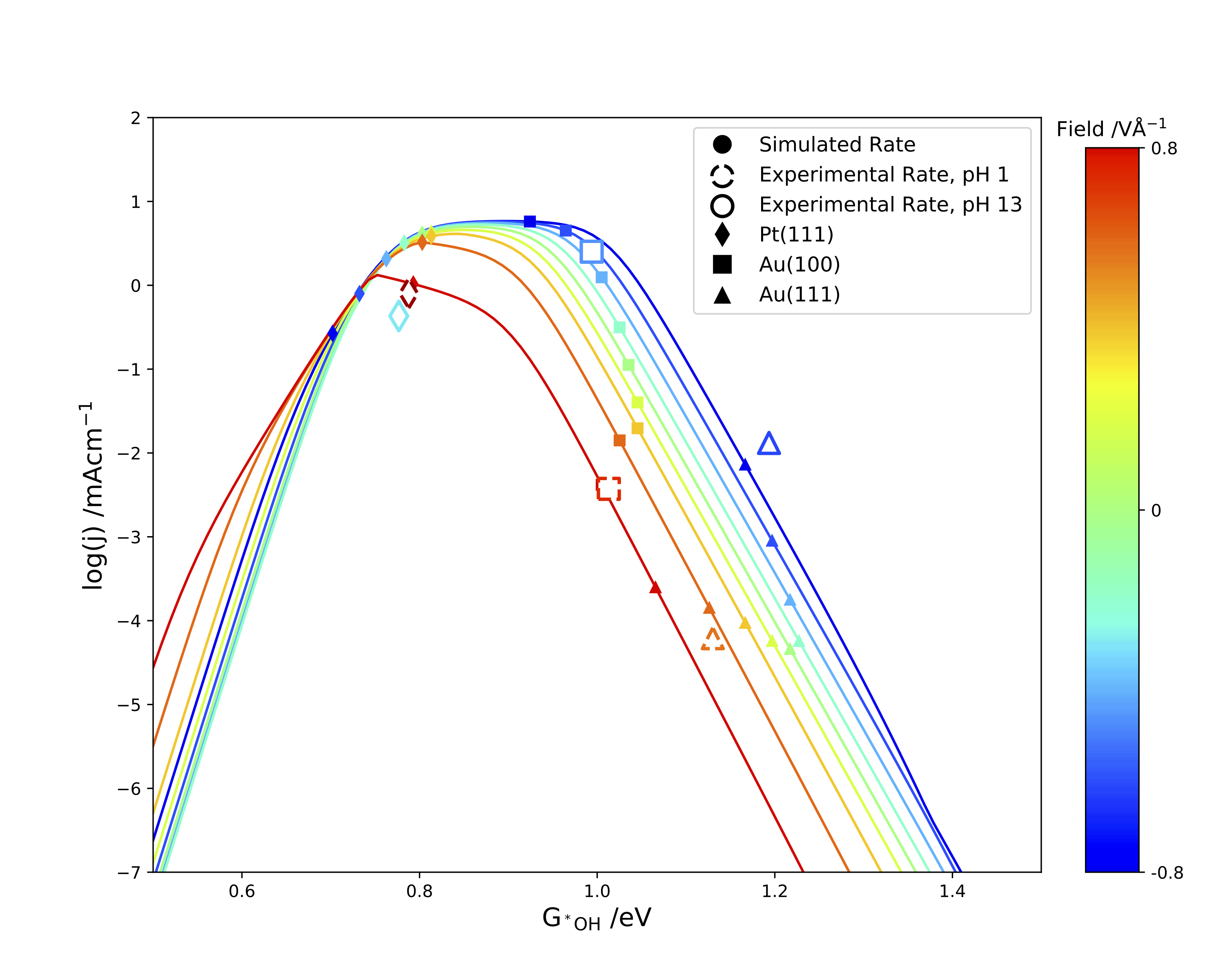
Topics
Checkout
This paper has an Extended Abstract file available; you must purchase the conference proceedings to access it.
Do you already own this?
Log In for instructions on accessing this content.
Pricing
Individuals
| AIChE Pro Members | $150.00 |
| AIChE Emeritus Members | $105.00 |
| AIChE Graduate Student Members | Free |
| AIChE Undergraduate Student Members | Free |
| AIChE Explorer Members | $225.00 |
| Non-Members | $225.00 |
