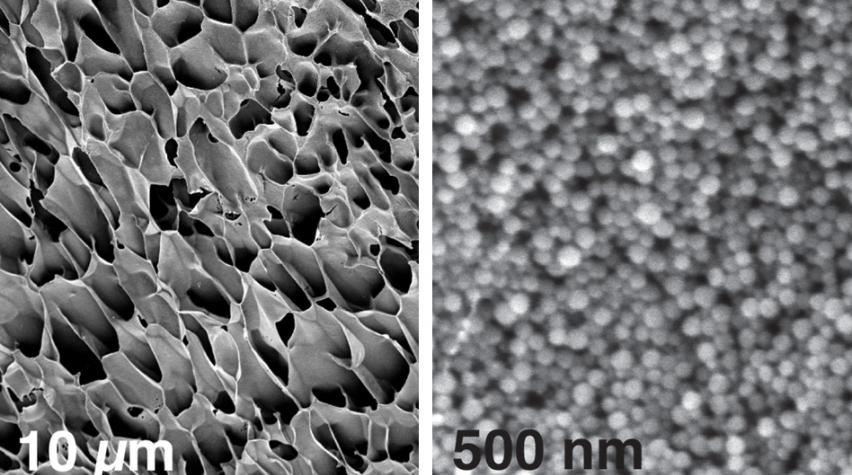
These scanning electron microscopy images, taken at different magnifications, show the structure of new hydrogels made of nanoparticles interacting with long polymer chains.
A team at Robert Langer's MIT lab has introduced a gel that can reassemble after being injected through a syringe. Until now, gels' malleability and potential for delivering drugs over time make them an incredible tool for medicine, but many current versions were not practical for all treatments because they had to be implanted surgically. The new gel opens new doors for treatment in a number of areas, including treatments for macular degeneration, heart disease, and various forms of cancer.
Simplicity of design
According to the report from MIT, the new gel consists of a mesh network made of two components: nanoparticles made of polymers entwined within strands of another polymer, such as cellulose.
This combination means the gel can undergo the stresses of being forced through a syringe and still reheal in the absence of those forces. In other words, it can enter the body through a syringe and reassemble, sidestepping the need for surgical implantation.
Advantages over predecessors
Previously devised hydrogels have relied on irreversible chemical linkages between polymers, which provide a durable material but not one whose shape can be altered once formed.
Other researchers have created such gels by engineering proteins that self-assemble into hydrogels, but this approach requires complex biochemical processes. The MIT team wanted to design something simpler. Their innovation combines two readily available components. One is a nanoparticle formed of PEG-PLA copolymers, first developed in Langer’s lab decades ago and now commonly used to package and deliver drugs. To form a hydrogel, the researchers mixed these particles with a polymer — in this case, cellulose.
Each polymer chain forms weak bonds with many nanoparticles, producing a loosely woven lattice of polymers and nanoparticles. Because each attachment point is fairly weak, the bonds break apart under mechanical stress, such as when injected through a syringe. When the shear forces are over, the polymers and nanoparticles form new attachments with different partners, healing the gel.
Works well with many drug types
The two components’ individual properties also allow for the delivery of both hydrophilic (such as proteins) and hydrophobic materials (such as small-molecule drugs, including chemotherapy drugs).
The researchers showed in their study that the gels survived injection under the skin of mice and successfully released two drugs, one hydrophobic and one hydrophilic, over several days.
For more information, see the MIT report and the published results of the study in Nature Communications.


